Abstract
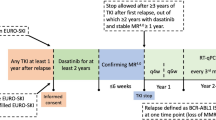
Limited data is available on the health-related quality of life (HRQoL) and symptoms of patients with chronic myeloid leukemia (CML) who are in treatment-free remission (TFR). We herein report HRQoL results from the EURO-SKI trial. Patients who had been on tyrosine kinase inhibitors (TKIs) therapy for at least 3 years and achieved MR4 for at least 1 year were enrolled from 11 European countries, and the EORTC QLQ-C30 and the FACIT-Fatigue questionnaires were used to assess HRQoL and fatigue respectively. Patients were categorized into the following age groups: 18–39, 40–59, 60–69 and ≥70 years. Of 728 patients evaluated at baseline, 686 (94%) completed HRQoL assessments. The median age at TKI discontinuation was 60 years. Our findings indicate that HRQoL and symptom trajectories may vary depending on specific age groups, with younger patients benefiting the most. Improvements in patients aged 60 years or older were marginal across several HRQoL and symptom domains. At the time of considering TKI discontinuation, physicians could inform younger patients that they may expect valuable HRQoL benefits. Considering the marginal improvements observed in patients aged 60 years or above, it may be important to further investigate the value of TFR compared to a lowest effective dose approach in this older group of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data underlying this article are available upon reasonable request to the corresponding author.
References
Kris MG, Benowitz SI, Adams S, Diller L, Ganz P, Kahlenberg MS, et al. Clinical cancer advances 2010: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2010;28:5327–47.
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35.
Branford S. Why is it critical to achieve a deep molecular response in chronic myeloid leukemia? Haematologica. 2020;105:2730–7.
Mikhaeel S, Atallah E. SOHO state of the art updates and next questions | update on treatment-free remission in chronic myeloid leukemia (CML). Clin Lymphoma Myeloma Leuk. 2023;23:333–9.
Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–47.
Schoenbeck KL, Flynn KE. Health-related quality of life of patients with chronic myeloid leukemia as measured by patient-reported outcomes: current state and future directions. Curr Hematol Malig Rep. 2021;16:491–9.
Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27:1511–9.
Guerin A, Chen L, Ionescu-Ittu R, Marynchenko M, Nitulescu R, Hiscock R, et al. Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res Opin. 2014;30:2317–28.
Richter J, Soderlund S, Lubking A, Dreimane A, Lotfi K, Markevarn B, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32:2821–3.
Park JS, Lee SE, Jeong SH, Jang EJ, Choi MY, Kim HJ, et al. Change of health-related profiles after Imatinib cessation in chronic phase chronic myeloid leukemia patients. Leuk Lymphoma. 2016;57:341–7.
Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–31.
Atallah E, Schiffer CA, Radich JP, Weinfurt KP, Zhang M-J, Pinilla-Ibarz J, et al. Assessment of outcomes after stopping tyrosine kinase inhibitors among patients with chronic myeloid leukemia. JAMA Oncol. 2021;7:42–50.
Schoenbeck KL, Atallah E, Lin L, Weinfurt KP, Cortes J, Deininger MWN, et al. Patient-reported functional outcomes in patients with chronic myeloid leukemia after stopping tyrosine kinase inhibitors. J Natl Cancer Inst. 2022;114:160–4.
Atallah E, Schiffer CA, Weinfurt KP, Zhang MJ, Radich JP, Oehler VG, et al. Design and rationale for the life after stopping tyrosine kinase inhibitors (LAST) study, a prospective, single-group longitudinal study in patients with chronic myeloid leukemia. BMC Cancer. 2018;18:359.
Efficace F, Baccarani M. Quality of life improvements in patients with chronic myeloid leukemia after stopping long-term therapy: who can benefit the most? J Natl Cancer Inst. 2022;114:9–11.
Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–57.
Mahon FX, Pfirrmann M, Dulucq S, Hochhaus A, Panayiotidis P, Almeida A, et al. European Stop Tyrosine Kinase Inhibitor Trial (EURO-SKI) in Chronic Myeloid Leukemia: Final Analysis and Novel Prognostic Factors for Treatment-Free Remission. J Clin Oncol. 2024: 42:1875–80 Jco2301647.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13:63–74.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual (3rd Edition). Published by: European Organisation for Research and Treatment of Cancer, Brussels 2001.
Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118:4554–60.
Hinz A, Singer S, Brahler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: Results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014;53:958–65.
Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96.
Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99.
Anota A, Hamidou Z, Paget-Bailly S, Chibaudel B, Bascoul-Mollevi C, Auquier P, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5–18.
Platzbecker U, Santini V, Fenaux P, Sekeres MA, Savona MR, Madanat YF, et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): a multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;403:248.
Schafer JL, Yucel RM. Computational strategies for multivariate linear mixed-effects models with missing values. J Computational Graph Stat. 2002;11:437–57. 2002/06/01
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Efficace F, Breccia M, Saussele S, Kossak-Roth U, Cardoni A, Caocci G, et al. Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? : GIMEMA and EORTC Quality of Life Group. Ann Hematol. 2012;91:1371–81.
Hyland KA, Eisel SL, Hoogland AI, Root JC, Bowles K, James B, et al. Cognition in patients treated with targeted therapy for chronic myeloid leukemia: a controlled comparison. Leuk Lymphoma. 2023;64:415–23.
Schoenbeck KL, Tummala S, Goyal NG, Smith CC, Shah NP. Cognitive dysfunction associated with tyrosine kinase inhibitors in patients with CMl in chronic phase. Blood. 2020;136:2–3. 2020/11/05/
Kota V, Atallah E. Musculoskeletal pain in patients with chronic myeloid leukemia after tyrosine kinase inhibitor therapy cessation. Clin Lymphoma Myeloma Leuk. 2019;19:480–7.
Flynn KE, Atallah E, Lin L, Shah NP, Silver RT, Larson RA, et al. Patient- and physician-reported pain after tyrosine kinase inhibitor discontinuation among patients with chronic myeloid leukemia. Haematologica. 2022;107:2641–9.
Berger MG, Pereira B, Rousselot P, Cony-Makhoul P, Gardembas M, Legros L, et al. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. Br J Haematol. 2019;187:337–46.
Lou J, Huang J, Wang Z, Wen B, Tu C, Huang W, et al. Chronic myeloid leukemia patients and treatment-free remission attitudes: a multicenter survey. Patient Prefer Adherence. 2018;12:1025–32.
Efficace F, Iurlo A, Patriarca A, Stagno F, Bee PC, Ector G, et al. Validation and reference values of the EORTC QLQ-CML24 questionnaire to assess health-related quality of life in patients with chronic myeloid leukemia. Leuk Lymphoma. 2021;62:669–78.
Acknowledgements
We thank all patients for dedicating their time to this study by completing Quality of Life questionnaires, and all participating centers and local research teams. We also thank Thomas Baldi and Francesco Sparano from the GIMEMA Central Office for their assistance.
Funding
The ELN Foundation supported the study. For the French part of the trial, financial support was given partially by the National Cancer Institute. Partly supported also by Czech Republic Ministry of Health-conceptual development of research organization (FNBr, 65269705). National Institute for Cancer Research Project (Program EXCELES, ID Project No. LX22NPO5102)-Funded by the European Union-Next Generation EU. This study was also partly supported by the GIMEMA Foundation.
Author information
Authors and Affiliations
Contributions
FE and SS conceptualized and designed the manuscript. AP, MC and FE performed the statistical analysis. FE, FXM, JR, HHH, MP and SS contributed to writing the manuscript. All authors (FE, FXM, JR, AP, MC, FEN, JM, DZ, JJWMJ, PP, HV, PK, AA, HHH, JML, UOS, AH, MGB, GE, HK, EF, PR, MP, SS) contributed to provision of study materials or patients, collection and assembly of data, data analysis and interpretation, critical review and approval of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Fabio Efficace had a consultancy or advisory role for AbbVie, Incyte, Novartis and JAZZ Pharmaceuticals, outside the submitted work. Franck E. Nicolini: Consultant for Novartis, Sun Pharma Ltd, board entity for Novartis, Incyte Biosciences, Pfizer, institutional grants from Novartis, Incyte Biosciences Europe. Daniela Zackova: served as a member of advisory board for Novartis, served on a speaker’s bureau for Novartis, Pfizer and Angelini, served as a consultant for Novartis and Angelini, and received travel grants from Novartis, Pfizer and Angelini. Andreas Hochhaus: Honoraria and research funding (Novartis, Incyte); research funding (Bristol Myers Squibb, Pfizer, MSD). Susanne Saussele: Honoraria: Novartis, Pfizer, Roche, Incyte. Research support, Novartis, BMS, Incyte.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in part at the 28th Annual Congress of the European Hematology (EHA) Association; June 8–11, 2023; Frankfurt, Germany
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Efficace, F., Mahon, FX., Richter, J. et al. Health-related quality of life and symptoms of chronic myeloid leukemia patients after discontinuation of tyrosine kinase inhibitors: results from the EURO-SKI Trial. Leukemia 38, 1722–1730 (2024). https://doi.org/10.1038/s41375-024-02341-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-024-02341-4