Abstract
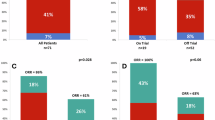
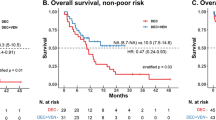
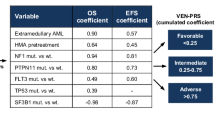
Optimal frontline use of active agents in T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL) is prudent to improve outcomes. We report the long-term follow-up of the phase 2 trial of HyperCVAD with nelarabine and pegylated asparaginase (Original cohort). In the latest protocol iteration venetoclax was added to the induction/consolidation regimen (Venetoclax cohort). Eligible patients were adults with untreated T-ALL/LBL or after minimal therapy and with adequate organ function. Primary endpoint of this analysis was improvement in 2-year progression free survival (PFS) and overall survival (OS) with venetoclax. From Aug 2007 to Dec 2024, 145 patients, at a median age of 35.4 years, were treated; 46 (33.8%) were in the venetoclax cohort. At median follow-up (mFU) of 62.4 months, 5-year PFS, duration of response (DOR), and OS were 63.7%, 72.0% and 66.2% respectively. In the venetoclax cohort (mFU 24.4 months) 2-year PFS (87.9% versus 64.1%, p = 0.03) and 2-year DOR (93.6% versus 69.2%, p = 0.005) were superior to the original cohort (mFU 89.4 months) and 2-year OS appeared better (87.8% versus 73.9%, p = 0.16). Febrile neutropenia was the most common serious adverse event, seen in 60% patients. The addition of venetoclax to HyperCVAD-nelarabine-pegylated asparaginase was tolerable and led to improvement in DOR and PFS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data available from corresponding author on reasonable request.
References
Dunsmore KP, Winter SS, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Children’s oncology group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38:3282–93.
Morita K, Jain N, Kantarjian H, Takahashi K, Fang H, Konopleva M, et al. Outcome of T-cell acute lymphoblastic leukemia/lymphoma: focus on near-ETP phenotype and differential impact of nelarabine. Am J Hematol. 2021;96:589–98.
Goekbuget N, Baumann A, Beck J, Brueggemann M, Diedrich H, Huettmann A, et al. PEG-asparaginase intensification in adult acute lymphoblastic leukemia (ALL): significant improvement of outcome with moderate increase of liver toxicity in the German multicenter study group for adult ALL (GMALL) study 07/2003. Blood. 2010;116:494.
Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4:1074–87.
Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11:1440–53.
Short NJ, Jabbour E, Jain N, Senapati J, Nasr L, Haddad FG, et al. A phase 1/2 study of mini-hyper-CVD plus venetoclax in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Adv. 2024;8:909–15.
Pinton A, Courtois L, Doublet C, Cabannes-Hamy A, Andrieu G, Smith C, et al. PHF6-altered T-ALL harbor epigenetic repressive switch at bivalent promoters and respond to 5-azacitidine and venetoclax. Clin Cancer Res. 2024;30:94–105.
Madero-Marroquin R, DuVall AS, Saygin C, Wang P, Gurbuxani S, Larson RA, et al. Durable responses in acute lymphoblastic leukaemia with the use of FLT3 and IDH inhibitors. Br J Haematol. 2024;204:1238–42.
Abaza Y M, Kantarjian H, Faderl S, Jabbour E, Jain N, Thomas D, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93:91–9.
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–32.
Jain N, Lamb AV, O’Brien S, Ravandi F, Konopleva M, Jabbour E, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127:1863–9.
Thomas DA, O’Brien S, Cortes J, Giles FJ, Faderl S, Verstovsek S, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–30.
Senapati J, Kantarjian H, Haddad FG, Short NJ, Welch MA, Jain N, Jabbour E. SOHO State of the Art Updates and Next Questions | Next Questions: Acute Lymphoblastic Leukemia. Clinical Lymphoma Myeloma and Leukemia. 2024;24:333–9.
Boissel N, Graux C, Huguet F, Pasquier F, Touzart A, Cluzeau T, et al. Frontline consolidation with nelarabine for adults with high-risk T-cell acute lymphoblastic leukemia. results of the Graall-2014/T Atriall phase 2 study. Blood. 2023;142:962.
Boddu PC, Senapati J, Ravandi-Kashani F, Jabbour EJ, Jain N, Ayres M, et al. A phase 1 study to evaluate the safety, pharmacology, and feasibility of continuous infusion nelarabine in patients with relapsed and/or refractory lymphoid malignancies. Cancer. 2023;129:580–9.
Acknowledgements
The data was partly presented at the American Society of Hematology Annual Meeting San Diego, 2023.
Funding
The study was supported by University of Texas MD Anderson Cancer Center Grant (CA016672), University of Texas MD Anderson SPORE (C1100632) and Charif Souki Cancer Research Fund.
Author information
Authors and Affiliations
Contributions
FR, JS and HMK designed the manuscript. JS, HB and RS collected the data. JS, and XH did the analysis. FR, JS, NJ, NJS, TK, GB, MK, WW, AM, GI, AF, FFM, YA, PK, HMK provided patients. FR and HMK supervised the study. JS wrote the first manuscript draft. All authors reviewed and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
FR has received research funding from Amgen, Astex Pharmaceuticals/Taiho Oncology, Bristol Myers Squibb/Celgene, Syos, AbbVie, Prelude, Xencor, Astellas Pharma, and Biomea Fusion; honoraria from Amgen, Bristol Myers Squibb/Celgene, Syos, AbbVie, and Astellas Pharma; has been a board of directors or advisory committee member for Astex Pharmaceuticals/Taiho Oncology; and has been a consultant for Bristol Myers Squibb/Celgene, Syos, Novartis, AbbVie, AstraZeneca, and Astellas Pharma. NJS has been a consultant for Takeda Oncology, AstraZeneca, Amgen, Novartis, and Pfizer; received research funding from Takeda Oncology, Astellas, and Stemline Therapeutics; and received honoraria from Amgen. TMK has been a consultant for AbbVie, Agios, Bristol Myers Squibb, Genentech, Jazz Pharmaceuticals, Novartis, Servier, and PinotBio; has received research funding from AbbVie, Bristol Myers Squibb, Genentech, Jazz Pharmaceuticals, Pfizer, Cellenkos, Ascentage Pharma, Galectin Therapeutics, Astellas Pharma, AstraZeneca, Amgen, Cyclacel Pharmaceuticals, Delta-Fly Pharma, Iterion Therapeutics, GlycoMimetics, and Regeneron Pharmaceuticals; and has received honoraria from Astex Pharmaceuticals. The remaining authors declare no competing financial interests. EJ has received research grants and consultancy from Amgen, Adaptive Biotechnologies, Ascentage, Autolus, Bristol-Myers Squibb, Pfizer, Takeda, Novartis, Abbvie, Genentech, ASTX, TGRX, TERN, and Johnson and Johnson. HMK has received research funding from AbbVie, Amgen, Ascentage Pharma, Bristol Myers Squibb, Daiichi Sankyo, ImmunoGen, Jazz Pharmaceuticals, and Novartis as well as honoraria from AbbVie, Amgen, Amphista Therapeutics, Ascentage Pharma, Astellas Pharma, Biologix, Curis, Ipsen, KAHR, Novartis, Pfizer, Precision BioSciences, Shenzhen TargetRx, and Takeda Oncology. All the other authors declare no relevant conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravandi, F., Senapati, J., Jain, N. et al. Longitudinal follow up of a phase 2 trial of venetoclax added to hyper-CVAD, nelarabine and pegylated asparaginase in patients with T-cell acute lymphoblastic leukemia and lymphoma. Leukemia 38, 2717–2721 (2024). https://doi.org/10.1038/s41375-024-02414-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-024-02414-4