Abstract
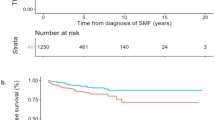
Hydroxyurea (HU) constitutes the first-line treatment in most patients with essential thrombocythemia (ET), but criteria for changing therapy are not clearly established. The prognostic value of complete hematological response (CHR) and resistance/intolerance to HU was assessed in 1080 patients from the Spanish Registry of ET, classified according to revised IPSET-Thrombosis stratification (Very low- n = 61, Low- n = 83, Intermediate- n = 261, and High-risk n = 675). With a median therapy duration of 5 years, CHR was registered in 720 (67%) patients (1-year probability 51%) and resistance/intolerance in 219 (20%) patients (5-years probability 13%). After correction by other risk factors, High-risk patients achieving CHR showed a reduced risk of arterial thrombosis (HR: 0.35, 95%CI: 0.2–0.6, p = 0.001) and a trend towards lower risk of venous thrombosis (HR: 0.45, 95%CI: 0.2–1.02, p = 0.06) whereas no association was observed for intermediate- or low-risk patients. In comparison with non-responders, intermediate- and high-risk patients achieving CHR had longer survival and lower myelofibrosis incidence. Development of resistance/intolerance to HU, mainly cytopenia, was associated with higher probability of myelofibrosis but no effect on survival or thrombotic risk was demonstrated. In conclusion, CHR with HU is associated with better outcomes and might be an early indicator for selecting candidates to second-line clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–13.
Cervantes F, Alvarez-Larran A, Talarn C, Gomez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–90.
Godfrey AL, Green A, Harrison CN. Essential thrombocythemia: challenges in clinical practice and future prospects. Blood. 2023;141:1943–53.
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70.
Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an international prognostic score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33.
Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369.
Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057–69.
Mesa RA, Jamieson C, Bhatia R, Deininger MW, Fletcher CD, Gerds AT, et al. NCCN guidelines insights: myeloproliferative neoplasms, version 2.2018. J Natl Compr Cancer Netw. 2017;15:1193–207.
Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113:4829–33.
Barosi G, Besses C, Birgegard G, Briere J, Cervantes F, Finazzi G, et al. A unified definition of clinical resistance/intolerance to hydroxyurea in essential thrombocythemia: results of a consensus process by an international working group. Leukemia. 2007;21:277–80.
Carobbio A, Finazzi G, Antonioli E, Vannucchi AM, Barosi G, Ruggeri M, et al. Hydroxyurea in essential thrombocythemia: rate and clinical relevance of responses by European LeukemiaNet criteria. Blood. 2010;116:1051–5.
Hernández-Boluda JC, Alvarez-Larrán A, Gómez M, Angona A, Amat P, Bellosillo B, et al. Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. 2011;152:81–8.
Alvarez-Larrán A, Sant’Antonio E, Harrison C, Kiladjian JJ, Griesshammer M, Mesa R, et al. Unmet clinical needs in the management of CALR-mutated essential thrombocythaemia: a consensus-based proposal from the European LeukemiaNet. Lancet Haematol. 2021;8:e658–65.
Kishtagari A, Gerds AT. Unmet need in essential thrombocythemia and polycythemia vera. Hematol Oncol Clin North Am. 2021;35:295–303.
Harrison CN, Nangalia J, Boucher R, Jackson A, Yap C, O’Sullivan J, et al. Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J Clin Oncol. 2023;41:3534–44.
Verstovsek S, Komatsu N, Gill H, Jin J, Lee SE, Hou HA, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022;18:2999–3009.
Harrison CN, Mead AJ, Panchal A, Fox S, Yap C, Gbandi E, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017;130:1889–97.
Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood. 2019;134:1498–509.
Gerds AT, Mesa R, Burke JM, Grunwald MR, Stein BL, Squier P, et al. Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL. Blood. 2024;143:1646–55.
Carobbio A, Ferrari A, Masciulli A, Ghirardi A, Barosi G, Barbui T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood Adv. 2019;3:1729–37.
Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian JJ, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120:1409–11.
Acknowledgements
We are indebted to all members of GEMFIN participating in the Spanish Registry of Essential thrombocythemia. We thank MFAR staff for their technical assistance.
Author information
Authors and Affiliations
Contributions
AAL designed the study, collected the data, performed the statistical analysis, analyzed and interpreted the results and wrote the paper. MS, MG, EAR and JCHB: collected the data, analyzed and interpreted the results, and wrote the paper. MSN, MPE, AS, RPL, FFM, GCT, GC, EM, PV, MACV, AM, AA, IPG, JMG, CGH, MIM, RS, MTGC, LF, BC, VGG, and AT collected the data, interpreted the results and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The Spanish registry of Essential thrombocythemia is financed with GEMFIN’s own funds without direct collaboration from any pharmaceutical company. This work has been funded by Instituto de Salud Carlos III (ISCIII) through the projects PI21/00231, PI21/00347, and PI21/00538 and co-funded by the European Union.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santaliestra, M., Garrote, M., Noya, M.S. et al. Prognostic value of response to first-line hydroxyurea according to IPSET stratification in essential thrombocythemia. Leukemia 38, 2636–2643 (2024). https://doi.org/10.1038/s41375-024-02416-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-024-02416-2