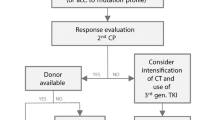
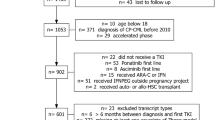
Abstract
The treatment strategy for children and adolescents with chronic myeloid leukemia in the chronic phase (CML-CP) has evolved from allogeneic hematopoietic stem cell transplantation (HSCT) to tyrosine kinase inhibitors (TKIs). With the advent of next-generation TKIs and new targeted therapies in the CML field, an international pediatric CML expert panel provides recommendations based on the medical literature (including previous pediatric guidelines), national standards, and treatment principles used in adults with CML-CP. Recommendations include diagnosis of the disease and details on managing the initial steps of care of children and adolescents with newly diagnosed CML-CP, including complications such as leukostasis. The treatment recommendations are based on the initiation of therapy with a first- or second-generation TKI according to the allocated European Treatment and Outcome Study (EUTOS) long-term survival score risk group of the patient. The subsequent steps are based on the results of recommended monitoring which can justify a switch to another TKI or a drug in development if there is resistance or toxicity. The panel also provides recommendations regarding the discontinuation criteria for TKIs in children and adolescents in sustained deep molecular response. Allogeneic HSCT is not recommended as the first-line of treatment for children with CML-CP but is to be considered in case of progression to the advanced phase or failure of several lines of treatment. The present treatment and management recommendations are intended to provide advice to clinicians in view of optimizing the care and the outcome of children and adolescents with CML-CP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Millot F, Suttorp M. Chronic myeloid Leukemia in children. In: Alaggio R, Chan JKC, Hasle H, editors. Haematolymphoid disorders—WHO classification book on paediatric tumours. 5th ed. Lyon: International Agency for Research on Cancer (IARC); 2023. p. 4–7.
Suttorp M, Millot F, Sembill S, Deutsch H, Metzler M. Definition, epidemiology, pathophysiology, and essential criteria for diagnosis of pediatric chronic myeloid leukemia. Cancers. 2021;13:798.
Ford M, Mauro M, Aftandilian C, Sakamoto KM, Hijiya N. Management of chronic myeloid leukemia in children and young adults. Curr Hematol Malig Rep. 2022;17:121–6.
de la Fuente J, Baruchel A, Biondi A, de Bont E, Dresse MF, Suttorp M, et al. International BFM Group (iBFM) Study Group Chronic Myeloid Leukaemia Committee Managing children with chronic myeloid leukaemia (CML): recommendations for the management of CML in children and young people up to the age of 18 years. Br J Haematol. 2014;167:33–47.
Athale U, Hijiya N, Patterson BC, Bergsagel J, Andolina JR, Bittencourt H, et al. Management of chronic myeloid leukemia in children and adolescents: recommendations from the Children’s Oncology Group CML Working Group. Pediatr Blood Cancer. 2019;66:e27827.
Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127:392–9.
Sembill S, Ampatzidou M, Chaudhury S, Dworzak M, Kalwak K, Karow A, et al. Management of children and adolescents with chronic myeloid leukemia in blast phase: International pediatric CML expert panel recommendations. Leukemia. 2023;37:505–17.
Hijiya N, Maschan A, Rizzari C, Shimada H, Dufour C, Goto H, et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia. Blood. 2019;134:2036–45.
Brivio E, Pennesi E, Willemse ME, Huitema ADR, Jiang Y, van Tinteren HDR, et al. Bosutinib in resistant and intolerant pediatric patients with chronic phase chronic myeloid leukemia: results from the phase I part of study ITCC054/COG AAML1921. J Clin Oncol. 2023;42:821–31.
Millot F, De Keizer J, Metzler M, Suttorp M, Sedlacek P, Luesink M, et al. Is the Eutos Long Term Survival (ELTS) score a useful marker to predict outcome in children with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP)? The experience of the International Registry of Childhood CML ; abstracts from the 2023 Annual Scientific Meeting of the American Society of Hematology (ASH). Blood. 2023;142:448.
Delehaye F, Rouger J, Brossier D, Suttorp M, Güneş AM, Sedlacek P, et al. Prevalence of anemia at diagnosis of pediatric chronic myeloid leukemia and prognostic impact on the disease course. Ann Hematol. 2023;102:563–70.
Knöfler R, Lange BS, Paul F, Tiebel O, Suttorp M. Bleeding signs due to acquired von Willebrand syndrome at diagnosis of chronic myeloid leukaemia in children. Br J Haematol. 2020;188:701–6.
Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29:2827–32.
Suttorp M, Schulze P, Glauche I, Göhring G, von Neuhoff N, Metzler M, et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: results from a phase III trial. Leukemia. 2018;32:1657–69.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumors: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84.
Berman E, Shah NP, Deninger M, Altman JK, Amaya M, Begna K, et al. CML and the WHO: Why? J Clin Oncol. 2024;42:984–6.
Millot F, Maledon N, Guilhot J, Güneş AM, Kalwak K, Suttorp M. Favourable outcome of de novo advanced phases of childhood chronic myeloid leukaemia. Eur J Cancer. 2019;115:17–23.
Millot F, Dupraz C, Guilhot J, Suttorp M, Brizard F, Leblanc T, et al. Additional cytogenetic abnormalities and variant t(9;22) at the diagnosis of childhood chronic myeloid leukemia: the experience of the International Registry for Chronic Myeloid Leukemia in Children and Adolescents. Cancer. 2017;123:3609–16.
Karow A, Göhring G, Sembill S, Lutterloh F, Neuhaus F, Callies S, et al. The cytogenetic landscape of pediatric chronic myeloid leukemia diagnosed in chronic phase. Cancers. 2022;14:1712.
Behrens YL, Gaschler L, Nienhold R, Reinkens T, Schirmer E, Knöß S, et al. Somatic variant profiling in chronic phase pediatric chronic myeloid leukemia. Haematologica. 2024;109:942–7.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in good-risk chronic granulocytic leukemia. Blood. 1984;63:789–99.
Hasford J, Baccarani M, Hoffman V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92.
Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56.
Salas DG, Glauche I, Tauer JT, Thiede C, Suttorp M. Can prognostic scoring systems for chronic myeloid leukemia as established in adults be applied to pediatric patients. Ann Hematol. 2015;94:1363–71.
Millot F, Guilhot J, Suttorp M, Meral Günes A, Sedlacek P, De Bont E, et al. Prognostic discrimination based on the EUTOS long-term survival score within the International Registry for Chronic Myeloid Leukemia in Children and Adolescents. Haematologica. 2017;102:1704–08.
Fischer MJ, Rheingold SR. Oncologic emergencies. In: Pizzo PA, Poplack DG, editors. Principles and practices of pediatric oncology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2011. p. 1125–51.
Kurosawa H, Tanizawa A, Tono C, Watanabe A, Shima H, Ito M, et al. Leukostasis in children and adolescents with chronic myeloid leukemia: Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer. 2016;63:406–11.
Millot F, Traore P, Guilhot J, Nelken B, Leblanc T, Leverger G, et al. Clinical and biological features at diagnosis in 40 children with chronic myeloid leukemia. Pediatrics. 2005;116:140–3.
Dou X, Zheng F, Zhang L, Jin J, Zhang Y, Liu B, et al. Adolescents experienced more treatment failure than children with chronic myeloid leukemia receiving imatinib as frontline therapy: a retrospective multicenter study. Ann Hematol. 2021;100:2215–28.
Roy Moulik N, Keerthivasagam S, Pandey A, Agiwale J, Hegde K, Chatterjee G, et al. Treatment and follow-up of children with chronic myeloid leukaemia in chronic phase (CML-CP) in the tyrosine kinase inhibitor (TKI) era—two decades of experience from the Tata Memorial Hospital paediatric CML (pCML) cohort. Br J Haematol. 2024;204:1249–61.
Suttorp M, Sembill S, Kalwak K, Metzler M, Millot F. Priapism at diagnosis of pediatric chronic myeloid leukemia: data derived from a large cohort of children and teenagers and a narrative review on priapism management. J Clin Med. 2023;12:4776.
Zhang D, Zhu Y, Jin Y, Kaweme NM, Dong Y. Leukapheresis and hyperleukocytosis, past and future. Int J Gen Med. 2021;14:3457–67.
Kumar S, Bansal D, Prakash M, Sharma P. Avascular necrosis of femoral head as the initial manifestation of CML. Pediatr Hematol Oncol. 2014;31:568–73.
Ericson C, Baird B, Broderick GA. Management of priapism: 2021 update. Urol Clin North Am. 2021;48:565–76.
Roy Moulik N, Harriss-Buchan A, Saglio G, Evans N, Suttorp M. Cases of patients treated in countries with limited resources and discussed by experts of the International CML Foundation (iCMLf); Case No. 1: a boy presenting with priapism and loss of vision. Case Rep Oncol Med. 2024:5534445.
Wang Y, Wang L, Xi Y, Li Z. Bleeding with negative coagulation screening test as initial presentation of chronic myelogenous leukemia managed by fresh frozen plasma: a case report. Medicine. 2019;98:e16984.
Aksu T, Erdem AY, Fettah A, Kaçar D, Avci Z, Yarali N, et al. Massive splenic infarction and portal vein thrombosis in children with chronic myeloid leukemia. J Pediatr Hematol Oncol. 2014;36:e471–2.
Drummond D, Lenoir M, Petit AY. Splenic infarction in a child revealing chronic myeloid leukemia. Eur J Pediatr. 2012;171:1141–2.
Giona F, Putti MC, Micalizzi C, Menna G, Moleti ML, Santoro N, et al. Long-term results of high-dose imatinib in children and adolescents with chronic myeloid leukaemia in chronic phase: the Italian experience. Br J Haematol. 2015;170:398–407.
Sadak K, Fultz K, Mendizaba A, Reaman G, Garcia-Gonzalez P, Levine PH. International patterns of childhood chronic myeloid leukemia: comparisons between the United States and resource-restricted Nations. Pediatr Blood Cancer. 2014;61:1774–78.
Suttorp M, Metzler M, Millot F, Shimada H, Bansal D, Meral Günes A, et al. Generic formulations of imatinib for treatment of Philadelphia chromosome-positive leukemia in pediatric patients. Pediatr Blood Cancer. 2018;65:e27431.
Gore L, Kearns PR, de Martino ML, Lee De Souza CA, Bertrand Y, Hijiya N, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36:1330–8.
Pennesi E, Brivio E, Willemse ME, de Boer EC, Jiang, Y, Van der Velden V, et al. One year analysis of growth data in newly-diagnosed pediatric patients with chronic myeloid leukemia treated with Bosutinib: results from trial ITCC-054/COG-AAML1921 (Abstract release date: 05/14/24) EHA Library. Pennesi E. 06/13/2024; 420781; P717.
Hochhaus H, Wang J, Kim DW, Kim DDH, Mayer J, Goh YT, et al. Asciminib in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2024;391:885–98.
Hijiya N, Shimada H, Kapoor S, Hoch M, Cardoso A, Dhamal V, et al. Pharmacokinetics and safety of asciminib (ASC) in pediatric patients (pts) with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP): interim results from the ASC4KIDS study (Abstract). J Clin Oncol. 2024;42:6562.
Lauseker M, Hanfstein B, Haferlach C, Schnittger S, Pfirrmann M, Fabarius A, et al. Equivalence of BCR-ABL transcript levels with complete cytogenetic remission in patients with chronic myeloid leukemia in chronic phase. J Cancer Res Clin Oncol. 2014;140:1965–9.
Branford S, Rudzki Z, Harper A, Grigg A, Taylor K, Durrant S, et al. Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Leukemia. 2003;1:2401–9.
Millot F, Guilhot J, Baruchel A, Petit A, Bertrand Y, Mazingue F, et al. Impact of early molecular response in children with chronic myeloid leukemia treated in the French Glivec phase 4 study. Blood. 2014;124:2408–10.
Johnson-Ansah H, Maneglier B, Huguet F, Legros L, Escoffre-Barbe M, Gardembas M, et al. Imatinib optimized therapy improves major molecular response rates in patients with chronic myeloid leukemia. Pharmaceutics. 2022;14:1676.
Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9.
Shelton DN, Bhagavatula P, Sepuvelda N, Beppu L, Gandhi S, Qin D, et al. Performance characteristics of the first Food and Drug Administration (FDA)-cleared digital droplet PCR (ddPCR) assay for BCR::ABL1 monitoring in chronic myelogenous leukemia. PLoS ONE. 2022;17. e0265278.
Chow EJ, Doody DR, Wilkes JJ, Becker LK, Chennupati S, Morin PE, et al. Adverse events among chronic myelogenous leukemia patients treated with tyrosine kinase inhibitors: a real-world analysis of health plan enrollees. Leuk Lymphoma. 2021;62:1203–10.
Janssen JM, Dorlo TPC, Steeghs N, Beijnen JH, Hanff LM, Van Eijkelenburg NKA, et al. Pharmacokinetic targets for therapeutic drug monitoring of small molecule kinase inhibitors in pediatric oncology. Clin Pharmacol Ther. 2020;108:494–505.
Zwaan CM, Rizzari C, Mechinaud F, Lancaster DL, Lehrnbecher T, van der Velden VH, et al. Dasatinib in children and adolescents with relapsed or refractory leukemia: results of the CA180-018 phase I dose-escalation study of the Innovative Therapies for Children with Cancer Consortium. J Clin Oncol. 2013;31:2460–8.
Hijiya N, Zwaan CM, Rizzari C, Foà R, Abbink F, Lancaster D, et al. Pharmacokinetics of nilotinib in pediatric patients with philadelphia chromosome-positive chronic myeloid leukemia or acute lymphoblastic leukemia. Clin Cancer Res. 2020;26:812–20.
Hijiya N, Maschan A, Rizzari C, Shimada H, Dufour C, Goto H, et al. A phase 2 study of nilotinib in pediatric patients with CML: long-term update on growth retardation and safety. Blood Adv. 2021;5:2925–34.
Hijiya N, Maschan A, Rizzari C, Shimada H, Dufour C, Goto H, et al. The long-term efficacy and safety of nilotinib in pediatric patients with CML: a 5-year update of the DIALOG study. Blood Adv. 2023;7:7279–89.
Champagne MA, Fu CH, Chang M, Chen H, Gerbing RB, Alonzo TA, et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57:56–62.
Yoo JW, Jo S, Ahn MB, Kim S, Lee JW, Kim M, et al. Front-line tyrosine kinase inhibitors in pediatric chronic myeloid leukemia: a study on efficacy and safety. Cancers. 2023;15:3862.
Aplenc R, Blaney SM, Strauss LC, Balis FM, Shusterman S, Ingle AM, et al. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children’s oncology group phase I consortium. J Clin Oncol. 2011;29:839–44.
Dong EE, Bruno MA, Kim JJ, Bernhardt MB, Brown AL, Gramatges MM. Distribution and frequency of tyrosine kinase inhibitor-associated long-term complications in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2022;69:e29786.
Rossoff J, Huynh V, Rau RE, Macy ME, Sulis ML, Schultz KR, et al. Experience with ponatinib in paediatric patients with leukaemia. Br J Haematol. 2020;189:363–8.
Millot F, Suttorp M, Versluys AB, Kalwak K, Nelken B, Ducassou S, et al. Ponatinib in childhood Philadelphia chromosome-positive leukaemias: an international registry of childhood chronic myeloid leukaemia study. Eur J Cancer. 2020;136:107–12.
Kodama Y, Sato A, Kato K, Sakaguchi H, Kato M, Kawasaki H, et al. Ponatinib in pediatric patients with Philadelphia chromosome-positive leukemia: a retrospective survey of the Japan Children’s Cancer Group. Int J Hematol. 2022;116:131–8.
Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Sig Transduct Target Ther. 2023;8:262.
Giona F, Mariani S, Gnessi L, Moleti ML, Rea M, De Vellis A, et al. Bone metabolism, growth rate and pubertal development in children with chronic myeloid leukemia treated with imatinib during puberty. Haematologica. 2013;98:e25.
Bansal D, Shava U, Varma N, Trehan A, Marwaha RK. Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer. 2012;59:481–4.
Tauer JT, Nowasz C, Sedlacek P, de Bont ES, Aleinikova OV, Suttorp M. Impairment of longitudinal growth by tyrosine kinase inhibitor (TKI) treatment-data from a large pediatric cohort with chronic myeloid leukemia (CML). Blood. 2014;124:522.
Shima H, Tokuyama M, Tanizawa A, Tono C, Hamamoto K, Muramatsu H, et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr. 2011;159:676–81.
Millot F, Guilhot J, Baruchel A, Petit A, Leblanc T, Bertrand Y, et al. Growth deceleration in children treated with imatinib for chronic myeloid leukaemia. Eur J Cancer. 2014;50:3206–11.
Gupta P, Banothu KK, Haldar P, Gupta AK, Meena JP. Effect of imatinib mesylate on growth in pediatric chronic myeloid leukemia: a systematic review and meta-analysis. J Pediatr Hematol Oncol. 2023;45:227–34.
Patterson BS, Samis J, Gore L, Zwaan CM, Sacchi M, Sy O, et al. Growth rate and endocrine effects of dasatinib therapy observed in a retrospective analysis of a phase 2 clinical trial for pediatric patients with chronic myeloid leukemia in chronic phase Abstract. HemaSphere. 2019;3:161–2.
Narayanan KR, Bansal D, Walia R, Sachdeva N, Bhansali A, Varma N, et al. Growth failure in children with chronic myeloid leukemia receiving imatinib is due to disruption of GH/IGF-1 axis. Pediatr Blood Cancer. 2013;60:1148–53.
Walia R, Aggarwal A, Bhansali A, Aggarwal A, Varma N, Sachdeva N, et al. Acquired neuro-secretory defect in growth hormone secretion due to Imatinib mesylate and the efficacy of growth hormone therapy in children with chronic myeloid leukemia. Pediatr Hematol Oncol. 2020;37:99–108.
Chang X, Zhou L, Chen X, Xu B, Cheng Y, Sun S, et al. Impact of imatinib on the fertility of male patients with chronic myelogenous leukaemia in the chronic phase. Target Oncol. 2017;12:827–32.
Christopoulos C, Dimakopoulou V, Rotas E. Primary ovarian insufficiency associated with imatinib therapy. N Engl J Med. 2008;358:1079–80.
Pallera A, Altman JK, Berman E, et al. NCCN guidelines insight—chronic myeloid leukemia. J Natl Compr Canc Netw. 2016;14:1505–12.
Pye SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–8.
Samis J, Lee P, Zimmerman D, Arceci RJ, Suttorp M, Hijiya N. Recognizing Endocrinopathies Associated With Tyrosine Kinase Inhibitor Therapy In Children With Chronic Myelogenous Leukemia. Pediatr Blood Cancer. 2016;63:1332.
Ganta RR, Nasaka S, Gundeti S. Impact of imatinib adherence on the cytogenetic response in pediatric chronic myeloid leukemia—chronic phase. Indian J Pediatr. 2016;8:1009–12.
Tan BK, Bee PC, Chua SS, Chen LC. Monitoring and improving adherence to tyrosine kinase inhibitors in patients with chronic myeloid leukemia: a systematic review. Patient Prefer Adherence. 2021;15:2563–75.
Suttorp M, Bornhäuser M, Metzler M, Millot F, Schleyer E. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Expert Rev Clin Pharmacol. 2018;11:219–31.
Giona F, Saglio G, Moleti ML, Piciocchi A, Rea M, Nanni M, et al. Treatment-free remission after imatinib discontinuation is possible in paediatric patients with chronic myeloid leukaemia. Br J Haematol. 2015;168:305–8.
Millot F, Suttorp M, Ragot S, Leverger G, Dalle JH, Thomas C, et al. Discontinuation of imatinib in children with chronic myeloid leukemia: a study from the International Registry of Childhood CML. Cancers. 2021;13:4102–9.
Shima H, Kada A, Tanizawa A, Sato I, Tono C, Ito M, et al. A Discontinuation of tyrosine kinase inhibitors in pediatric chronic myeloid leukemia. Pediatr Blood Cancer. 2022;69:e29699.
Roy Moulik N, Keerthivasagam S, Chatterjee G, Agiwale J, Rane P, Dhamne C, et al. Treatment-free remissions in children with chronic myeloid leukemia (CML): a prospective study from the Tata Memorial Hospital (TMH) pediatric CML (pCML) cohort. Am J Hematol. 2024;100:210–7.
Millot F, Esperou H, Bordigoni P, Dalle JH, Michallet M, Michel G, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in childhood: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Bone Marrow Transpl. 2003;32:993–9.
Cwynarski K, Roberts IA, Iacobelli S, Van Biezen A, Brand R, Devergie A, et al. Stem cell transplantation for chronic myeloid leukemia in children. Blood. 2003;102:1224–31.
Muramatsu H, Kojima S, Yoshimi A, Atsuta Y, Kato K, Nagatoshi Y, et al. Outcome of 125 children with chronic myelogenous leukemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Biol Blood Marrow Transpl. 2010;16:231–8.
Suttorp M, Claviez A, Bader P, Peters C, Gadner H, Ebell W, et al. Allogeneic stem cell transplantation for pediatric and adolescent patients with CML: results from the prospective trial CML-paed I. Klin Padiatr. 2009;221:351–7.
Kurosawa H, Tanizawa A, Muramatsu H, Tono C, Watanabe A, Shima H, et al. Sequential use of second-generation tyrosine kinase inhibitors following imatinib therapy in pediatric chronic myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer. 2018;65:e27368.
Millot F, Duche J, Günes AM, Kalwak K, Metzler M, Suttorp M et al. Hematopoietic stem cell allograft in children and adolescents with chronic myeloid leukemia in chronic phase: the experience of the International registry for childhood chronic myeloid leukemia (I-CML-Ped Study). Abstract from the 2024 Annual Scientific Meeting of the International Society of Pediatric Oncology (SIOP). Pediatric Blood Cancer. 2024;71:134.
Chaudhury S, Sparapani R, Hu ZH, Nishihori T, Abdel-Azim H, Malone A, et al. Outcomes of allogeneic hematopoietic cell transplantation in children and young adults with chronic myeloid leukemia: a CIBMTR cohort analysis. Biol Blood Marrow Transpl. 2016;22:1056–64.
Hafez HA, Abdallah A, Hammad M, Hamdy N, Yassin D, Salem S, et al. Outcomes of allogenic hematopoietic cell transplantation for childhood chronic myeloid leukemia: single-center experience. Pediatr Transpl. 2020;24:e13664.
Shimada H, Tanizawa A, Kondo T, Nagamura-Inoue T, Yasui M, Tojo A, et al. Prognostic factors for outcomes of allogeneic HSCT for children and adolescents/young adults with CML in the TKI era. Transpl Cell Ther. 2022;28:376–89.
Pichler H, Sedlacek P, Meisel R, Beier R, Faraci M, Kalwak K, et al. Haematopoietic stem cell transplantation after reduced intensity conditioning in children and adolescents with chronic myeloid leukaemia: a prospective multicentre trial of the I-BFM Study Group. Br J Haematol. 2024;205:268–79.
Broglie L, Hijiya N, Helenowski IB, Dilley K, Schneiderman J, Tse W, et al. Long-term follow-up of children with chronic myeloid leukemia after hematopoietic stem cell transplantation and tyrosine kinase inhibitor therapy. Leuk Lymphoma. 2016;57:949–52.
Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685–91.
Suttorp M, Carrion A, Hijiya N. Chronic myeloid leukemia in children: immune function and vaccinations. J Clin Med. 2021;10:4056.
de Lavallade H, Khoder A, Hart M, Sarvaria A, Sekine T, Alsuliman A, et al. Tyrosine kinase inhibitors impair B-cell immune responses in CML through off-target inhibition of kinases important for cell signaling. Blood. 2013;122:227–38.
Bettoni da Cunha-Riehm C, Hildebrand V, Nathrath M, Metzler M, Suttorp M. Vaccination with live attenuated vaccines in four children with chronic myeloid leukemia while on imatinib treatment. Front Immunol. 2020;11:628.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
Cross NCP, Ernst T, Branford S, Cayuela JM, Deininger M, Fabarius A, et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia. 2023;37:2150–67.
Acknowledgements
The authors gratefully acknowledge Professor Irene Roberts (emeritus professor of pediatric hematology, MRC Weatherall Institute of Molecular Medicine, Oxford University, United Kingdom) and Professor François Guilhot (emeritus professor of hematology, University of Poitiers, France) for helpful comments on the manuscript. The manuscript was reread and reviewed by Jeffrey Arsham, the American medical proofreader at the University Hospital of Poitiers, France.
Author information
Authors and Affiliations
Contributions
FM, MS, and MM designed the project and defined items for the panel discussion. FM, MA, NRM, ST, AE, MH, HP, TL, AT, HS, WA, WY, AK, RF, ML, MD, SS, BDM, PS, KRS, KK, BV, UA, NH, MM, and MS contributed to the literature review, to the discussion and the detailed recommendations. FM drafted the manuscript. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Millot, F., Ampatzidou, M., Moulik, N.R. et al. Management of children and adolescents with chronic myeloid leukemia in chronic phase: International pediatric chronic myeloid leukemia expert panel recommendations. Leukemia 39, 779–791 (2025). https://doi.org/10.1038/s41375-025-02543-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02543-4
This article is cited by
-
Chronische myeloische Leukämie bei Kindern und Jugendlichen
Die Onkologie (2025)
-
2025 European LeukemiaNet recommendations for the management of chronic myeloid leukemia
Leukemia (2025)