Abstract
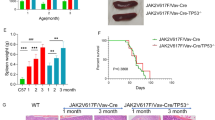
Therapy with pegylated interferon alpha (pegIFNα) can induce a deep molecular response in a subset of patients with myeloproliferative neoplasms (MPN). Here we investigated the role of Socs2, a negative regulator of cytokine signaling, in modulating the response to pegIFNα in a JAK2-V617F mouse model of MPN. Deleting Socs2 in JAK2-V617F mice resulted in increased sensitivity to cytokines, without causing significant alterations in the MPN phenotype. When subjected to pegIFNα, the loss of Socs2 enhanced the depletion of JAK2-mutant hematopoietic stem cells (HSCs), evidenced by reduced chimerism in peripheral blood and bone marrow compared to vehicle controls. Additionally, pegIFNα-treated Socs2-deficient JAK2-mutant HSCs exhibited functional impairments in secondary transplantations, reflecting long-term detrimental decline of their stemness. These findings demonstrate that loss of Socs2 enhances the effectiveness of pegIFNα in depleting the JAK2-mutant HSC clone. In line with the genetic ablation of Socs2, the SOCS2 inhibitor MN714 combined with IFNα exhibited better efficacy than IFNα alone in reducing the output of CD34+ cells from PV patients in vitro. Targeting SOCS2 could therefore improve therapeutic responsiveness in MPN patients receiving interferon therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Luque Paz D, Kralovics R, Skoda RC. Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood. 2023;141:1909–21.
Kiladjian JJ, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia. 2022;36:1408–11.
Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. 2023;37:2129–32.
Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8.
Demerdash Y, Kain B, Essers MAG, King KY. Yin and Yang: The dual effects of interferons on hematopoiesis. Exp Hematol. 2021;96:1–12.
Pietras EM, Lakshminarasimhan R, Techner JM, Fong S, Flach J, Binnewies M, et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J Exp Med. 2014;211:245–62.
Hasan S, Lacout C, Marty C, Cuingnet M, Solary E, Vainchenker W, et al. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNalpha. Blood. 2013;122:1464–77.
Mullally A, Bruedigam C, Poveromo L, Heidel FH, Purdon A, Vu T, et al. Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-alpha in a murine model of polycythemia vera. Blood. 2013;121:3692–702.
Rao TN, Hansen N, Stetka J, Luque Paz D, Kalmer M, Hilfiker J, et al. JAK2-V617F and interferon-alpha induce megakaryocyte-biased stem cells characterized by decreased long-term functionality. Blood. 2021;137:2139–51.
Chen E, Ahn JS, Massie CE, Clynes D, Godfrey AL, Li J, et al. JAK2V617F promotes replication fork stalling with disease-restricted impairment of the intra-S checkpoint response. Proc Natl Acad Sci USA. 2014;111:15190–5.
Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–52.
Austin RJ, Straube J, Bruedigam C, Pali G, Jacquelin S, Vu T, et al. Distinct effects of ruxolitinib and interferon-alpha on murine JAK2V617F myeloproliferative neoplasm hematopoietic stem cell populations. Leukemia. 2020;34:1075–89.
Keewan E, Matlawska-Wasowska K The Emerging Role of Suppressors of Cytokine Signaling (SOCS) in the development and progression of leukemia. Cancers. 2021;13:4000–20.
Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462:1–13.
Nguyen CH, Gluxam T, Schlerka A, Bauer K, Grandits AM, Hackl H, et al. SOCS2 is part of a highly prognostic 4-gene signature in AML and promotes disease aggressiveness. Sci Rep. 2019;9:9139.
Rodriguez-Fraticelli AE, Weinreb C, Wang SW, Migueles RP, Jankovic M, Usart M, et al. Single-cell lineage tracing unveils a role for TCF15 in haematopoiesis. Nature. 2020;583:585–9.
Hansen N, Agerstam H, Wahlestedt M, Landberg N, Askmyr M, Ehinger M, et al. SOCS2 is dispensable for BCR/ABL1-induced chronic myeloid leukemia-like disease and for normal hematopoietic stem cell function. Leukemia. 2013;27:130–5.
Vitali C, Bassani C, Chiodoni C, Fellini E, Guarnotta C, Miotti S, et al. SOCS2 Controls proliferation and stemness of hematopoietic cells under stress conditions and its deregulation marks unfavorable acute leukemias. Cancer Res. 2015;75:2387–99.
Quentmeier H, Geffers R, Jost E, Macleod RA, Nagel S, Rohrs S, et al. SOCS2: inhibitor of JAK2V617F-mediated signal transduction. Leukemia. 2008;22:2169–75.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40.
Gothert JR, Gustin SE, Hall MA, Green AR, Gottgens B, Izon DJ, et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood. 2005;105:2724–32.
Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–22.
Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, et al. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–73.
Kubovcakova L, Lundberg P, Grisouard J, Hao-Shen H, Romanet V, Andraos R, et al. Differential effects of hydroxyurea and INC424 on mutant allele burden and myeloproliferative phenotype in a JAK2-V617F polycythemia vera mouse model. Blood. 2013;121:1188–99.
Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15.
Usart M, Stetka J, Luque Paz D, Hansen N, Kimmerlin Q, Almeida Fonseca T, et al. Loss of Dnmt3a increases self-renewal and resistance to pegIFN-alpha in JAK2-V617F-positive myeloproliferative neoplasms. Blood. 2024;143:2490–503.
Ramachandran S, Ciulli A. Building ubiquitination machineries: E3 ligase multi-subunit assembly and substrate targeting by PROTACs and molecular glues. Curr Opin Struct Biol. 2021;67:110–9.
Ramachandran S, Makukhin N, Haubrich K, Nagala M, Forrester B, Lynch DM, et al. Structure-based design of a phosphotyrosine-masked covalent ligand targeting the E3 ligase SOCS2. Nat Commun. 2023;14:6345.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Lun ATL, Marioni JC. Overcoming confounding plate effects in differential expression analyses of single-cell RNA-seq data. Biostatistics. 2017;18:451–64.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25.
Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, Panero R, Patel SH, Jankovic M, et al. Clonal analysis of lineage fate in native haematopoiesis. Nature. 2018;553:212–6.
Tan G, Wolski WE, Kummer S, Hofstetter M, Theocharides APA, Manz MG, et al. Proteomic identification of proliferation and progression markers in human polycythemia vera stem and progenitor cells. Blood Adv. 2022;6:3480–93.
Etienne A, Carbuccia N, Adelaide J, Bekhouche I, Remy V, Sohn C, et al. Rearrangements involving 12q in myeloproliferative disorders: possible role of HMGA2 and SOCS2 genes. Cancer Genet Cytogenet. 2007;176:80–8.
Unni AM, Harbourne B, Oh MH, Wild S, Ferrarone JR, Lockwood WW, et al. Hyperactivation of ERK by multiple mechanisms is toxic to RTK-RAS mutation-driven lung adenocarcinoma cells. Elife. 2018;7.
James NE, Beffa L, Oliver MT, Borgstadt AD, Emerson JB, Chichester CO, et al. Inhibition of DUSP6 sensitizes ovarian cancer cells to chemotherapeutic agents via regulation of ERK signaling response genes. Oncotarget. 2019;10:3315–27.
Dias MH, Bernards R. Playing cancer at its own game: activating mitogenic signaling as a paradoxical intervention. Mol Oncol. 2021;15:1975–85.
Acknowledgements
We thank the Flow Cytometry core facility of the Department of Biomedicine for help in cell sorting and flow cytometry analysis and the Genomics Facility of the University of Basel for the help with RNA preparation for single-cell and bulk RNAseq and members of the laboratory for helpful discussions and critical reading of our manuscript.
Funding
This work was supported by grants from the Swiss National Science Foundation (31003A_166613, 310030_185297/1, and 310030_185297/2), the Swiss Cancer Research foundation (KFS-3655-02-2015 and KFS-4462-02-2018), the Stiftung für Hämatologische Forschung, and the Cancer Prevention and Research Institute of Texas (CPRIT) RR240024 to RCS, and a grant from the Research Fund of the University of Basel for Junior Researchers to QK. Work in the A.C. Lab was supported by the Innovative Medicines Initiative 2 (IMI2) Joint Undertaking under grant agreement no. 875510 (EUbOPEN project). The IMI2 Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program, European Federation of Pharmaceutical Industries and Associations (EFPIA) companies, and associated partners KTH, OICR, Diamond, and McGill.
Author information
Authors and Affiliations
Contributions
MU designed and performed research, analyzed data and wrote the manuscript; QK, JS, CS, RK, SR, TAF and HHS performed research and analyzed data; JR and AET analyzed data, NM, DL and AC developed MN714 and analyzed data, and RCS designed research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.C.S. is a scientific advisor/SAB member and has equity in Ajax Therapeutics, he is a co-founder of MPNquest and he consulted for and/or received honoraria from Novartis, BMS/Celgene, AOP, GSK, Baxalta and Pfizer. M.U. is a co-founder of MPNquest. A.C. is a scientific founder and shareholder of Amphista Therapeutics, a company that is developing targeted protein degradation therapeutic platforms. The Ciulli laboratory receives or has received sponsored research support from Almirall, Amgen, Amphista Therapeutics, Boehringer Ingelheim, Eisai, Merck KaaG, Nurix Therapeutics, Ono Pharmaceutical and Tocris-Biotechne.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Usart, M., Kimmerlin, Q., Stetka, J. et al. Loss of Socs2 improves molecular responses to IFNα in a mouse model of myeloproliferative neoplasms driven by JAK2-V617F. Leukemia 39, 876–887 (2025). https://doi.org/10.1038/s41375-025-02550-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02550-5