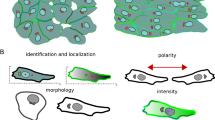
Abstract
Cell polarity, the asymmetric organization of cellular components, is evolutionarily conserved from unicellular and multicellular organisms and is crucial for many biological processes. Polarity is required to maintain cell and tissue integrity by regulating cell division, migration, orientation, cell-cell interactions, and morphogenesis. Impaired polarity leads to dysregulation of cellular functions and is associated with disease. Understanding how polarity is established, maintained, and regulated is thus critical to improving our knowledge of pathologies and devising novel therapies. Here, we explore the various manifestations of cell polarity across different model systems, tissues, and cell types and focus on known polarity mechanisms in hematopoietic stem and progenitor cells. We discuss how cells with vastly different functions utilize conserved molecular complexes to establish cell polarity while adapting polarity proteins to unique cell-type-specific functions. In this discussion, we attempt to extract common themes and concepts to improve our understanding of cell polarity in hematological malignancies and other diseases. Finally, we summarize, compare, and evaluate classical as well as recently developed methods to quantify cell polarity, highlight important advances in imaging and analytical techniques, and suggest critical next steps required to move the field forward.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Goehring NW, Grill SW. Cell polarity: mechanochemical patterning. Trends Cell Biol. 2013;23:72–80.
Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–74.
Treuner-Lange A, Søgaard-Andersen L. Regulation of cell polarity in bacteria. J Cell Biol. 2014;206:7–17.
Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol cell Biol. 2014;15:225–42.
Piroli ME, Blanchette JO, Jabbarzadeh E. Polarity as a physiological modulator of cell function. Front Biosci. 2019;24:451.
Raman R, Pinto CS, Sonawane M. Polarized organization of the cytoskeleton: regulation by cell polarity proteins. J Mol Biol. 2018;430:3565–84.
Gubieda AG, Packer JR, Squires I, Martin J, Rodriguez J. Going with the flow: insights from Caenorhabditis elegans zygote polarization. Philos Trans R Soc B. 2020;375:20190555.
Llense F, Etienne-Manneville S. Front-to-Rear Polarity in Migrating Cells. In: Ebnet K, editor. Cell Polarity 1: Biological Role and Basic Mechanisms. Cham: Springer International Publishing; 2015. p. 115–46.
Campanale JP, Sun TY, Montell DJ. Development and dynamics of cell polarity at a glance. J Cell Sci. 2017;130:1201–7.
Butler MT, Wallingford JB. Planar cell polarity in development and disease. Nat Rev Mol Cell Biol. 2017;18:375–88.
Chiou J-g, Balasubramanian MK, Lew DJ. Cell polarity in yeast. Annu Rev Cell Dev Biol. 2017;33:77–101.
Buckley CE, St Johnston D. Apical–basal polarity and the control of epithelial form and function. Nat Rev Mol Cell Biol. 2022;23:559–77.
Mohr J, Dash BP, Schnoeder TM, Wolleschak D, Herzog C, Tubio Santamaria N, et al. The cell fate determinant Scribble is required for maintenance of hematopoietic stem cell function. Leukemia. 2018;32:1211–21.
Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–30.
Zjablovskaja P, Florian MC. Acute myeloid leukemia: aging and epigenetics. Cancers. 2019;12:103.
Carolina Florian M, Geiger H. Concise review: polarity in stem cells, disease, and aging. Stem cells. 2010;28:1623–9.
Mejia-Ramirez E, Geiger H, Florian MC. Loss of epigenetic polarity is a hallmark of hematopoietic stem cell aging. Hum Mol Genet. 2020;29:R248–R54.
Woods B, Lew DJ. Polarity establishment by Cdc42: Key roles for positive feedback and differential mobility. Small GTPases. 2019;10:130–7.
Goryachev AB, Pokhilko AV. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 2008;582:1437–43.
McCaffrey LM, Macara IG. Signaling pathways in cell polarity. Cold Spring Harb Perspect Biol. 2012;4:a009654.
Mei K, Guo W. The exocyst complex. Curr Biol. 2018;28:R922–R5.
Skau CT, Courson DS, Bestul AJ, Winkelman JD, Rock RS, Sirotkin V, et al. Actin filament bundling by fimbrin is important for endocytosis, cytokinesis, and polarization in fission yeast. J Biol Chem. 2011;286:26964–77.
Bendezu FO, Vincenzetti V, Martin SG. Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PloS one. 2012;7:e40248.
Piel M, Tran PT. Cell shape and cell division in fission yeast. Curr Biol. 2009;19:R823–R7.
Díaz-Díaz C, Baonza G, Martín-Belmonte F. The vertebrate epithelial apical junctional complex: Dynamic interplay between Rho GTPase activity and cell polarization processes. Biochim et Biophys Acta (BBA)-Biomemb. 2020;1862:183398.
Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, et al. Yurt, Coracle, Neurexin IV and the Na+, K+-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–5.
Riga A, Castiglioni VG, Boxem M. New insights into apical-basal polarization in epithelia. Curr Opin Cell Biol. 2020;62:1–8.
Osswald M, Barros-Carvalho A, Carmo AM, Loyer N, Gracio PC, Sunkel CE, et al. aPKC regulates apical constriction to prevent tissue rupture in the Drosophila follicular epithelium. Curr Biol. 2022;32:4411–27.e8.
Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat cell Biol. 2012;14:1235–43.
Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–6.
Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev cell. 2004;7:413–24.
Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol. 2006;8:978–85.
Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development. 2006;133:3507–16.
Wallenfang MR, Seydoux G. Polarization of the anterior–posterior axis of C. elegans is a microtubule-directed process. Nature. 2000;408:89–92.
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9.
Polgar N, Fogelgren B. Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb Perspect Biol. 2018;10:a031401.
Paul NR, Jacquemet G, Caswell PT. Endocytic trafficking of integrins in cell migration. Curr Biol. 2015;25:R1092–R105.
Daulat AM, Borg J-P. Wnt/planar cell polarity signaling: new opportunities for cancer treatment. Trends cancer. 2017;3:113–25.
Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33.
Loeffler D, Wehling A, Schneiter F, Zhang Y, Müller-Bötticher N, Hoppe PS, et al. Asymmetric lysosome inheritance predicts activation of haematopoietic stem cells. Nature. 2019;573:426–9.
Loeffler D, Schneiter F, Wang W, Wehling A, Kull T, Lengerke C, et al. Asymmetric organelle inheritance predicts human blood stem cell fate. Blood, J Am Soc Hematol. 2022;139:2011–23.
Mastrogiovanni M, Di Bartolo V, Alcover A. Cell polarity regulators, multifunctional organizers of lymphocyte activation and function. Biomed J. 2022;45:299–309.
Molon B, Liboni C, Viola A. CD28 and chemokine receptors: Signalling amplifiers at the immunological synapse. Front Immunol. 2022;13:938004.
Bashour KT, Tsai J, Shen K, Lee J-H, Sun E, Milone MC, et al. Cross talk between CD3 and CD28 is spatially modulated by protein lateral mobility. Mol Cell Biol. 2014;34:955–64.
Capitani N, Baldari CT. The immunological synapse: an emerging target for immune evasion by bacterial pathogens. Front Immunol. 2022;13:943344.
Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–6.
Huse M, Le Floc’h A, Liu X. From lipid second messengers to molecular motors: microtubule‐organizing center reorientation in T cells. Immunol Rev. 2013;256:95–106.
Chauveau A, Le Floc’h A, Bantilan NS, Koretzky GA, Huse M. Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci Signal. 2014;7:ra82–ra.
Borsa M, Barnstorf I, Baumann NS, Pallmer K, Yermanos A, Gräbnitz F, et al. Modulation of asymmetric cell division as a mechanism to boost CD8+ T cell memory. Sci Immunol. 2019;4:eaav1730.
Bessy T, Candelas A, Souquet B, Saadallah K, Schaeffer A, Vianay B, et al. Hematopoietic progenitors polarize in contact with bone marrow stromal cells in response to SDF1. J Cell Biol. 2021;220.
Wehling A, Loeffler D, Zhang Y, Kull T, Donato C, Szczerba B, et al. Combining single-cell tracking and omics improves blood stem cell fate regulator identification. Blood J Am Soc Hematol. 2022;140:1482–95.
Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–65.
Hernández-Malmierca P, Vonficht D, Schnell A, Uckelmann HJ, Bollhagen A, Mahmoud MA, et al. Antigen presentation safeguards the integrity of the hematopoietic stem cell pool. Cell Stem Cell. 2022;29:760–75.e10.
Loeffler D, Schroeder T. Symmetric and asymmetric activation of hematopoietic stem cells. Curr Opin Hematol. 2021;28:262–8.
de Haan G, Lazare SS. Aging of hematopoietic stem cells. Blood J Am Soc Hematol. 2018;131:479–87.
Umbayev B, Safarova Y, Yermekova A, Nessipbekova A, Syzdykova A, Askarova S. Role of a small GTPase Cdc42 in aging and age-related diseases. Biogerontology. 2023;24:27–46.
Florian MC, Nattamai KJ, Dörr K, Marka G, Überle B, Vas V, et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503:392–6.
Amoah A, Keller A, Emini R, Hoenicka M, Liebold A, Vollmer A, et al. Aging of human hematopoietic stem cells is linked to changes in Cdc42 activity. Haematologica. 2022;107:393.
Liu W, Du W, Shang X, Wang L, Evelyn C, Florian MC, et al. Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia. 2019;33:749–61.
Pichaud F, Walther RF, Nunes de Almeida F. Regulation of Cdc42 and its effectors in epithelial morphogenesis. J Cell Sci. 2019;132:jcs217869.
Mei Y, Han X, Liu Y, Yang J, Sumagin R, Ji P. Diaphanous-related formin mDia2 regulates beta2 integrins to control hematopoietic stem and progenitor cell engraftment. Nat Commun. 2020;11:3172.
Kandi R, Senger K, Grigoryan A, Soller K, Sakk V, Schuster T, et al. Cdc42‐Borg4‐Septin7 axis regulates HSC polarity and function. EMBO Rep. 2021;22:e52931.
Mejia-Ramirez E, Florian MC. Understanding intrinsic hematopoietic stem cell aging. Haematologica. 2020;105:22.
Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, et al. LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol. 2018;19:1–21.
Heidel FH, Bullinger L, Arreba-Tutusaus P, Wang Z, Gaebel J, Hirt C, et al. The cell fate determinant Llgl1 influences HSC fitness and prognosis in AML. J Exp Med. 2013;210:15–22.
Althoff MJ, Nayak RC, Hegde S, Wellendorf AM, Bohan B, Filippi M-D, et al. Yap1-Scribble polarization is required for hematopoietic stem cell division and fate. Blood, J Am Soc Hematol. 2020;136:1824–36.
Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–30.
Heinonen KM, Vanegas JR, Lew D, Krosl J, Perreault C. Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. PloS One. 2011;6:e19279.
Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, et al. Atypical protein kinase C (aPKCζ and aPKCλ) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci. 2011;108:9957–62.
Loeffler D, Schroeder T. Understanding cell fate control by continuous single-cell quantification. Blood J Am Soc Hematol. 2019;133:1406–14.
Suda T, Suda J, Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci. 1984;81:2520–4.
Takano T, Xu C, Funahashi Y, Namba T, Kaibuchi K. Neuronal polarization. Development. 2015;142:2088–93.
Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–26.
Florian MC, Klose M, Sacma M, Jablanovic J, Knudson L, Nattamai KJ, et al. Aging alters the epigenetic asymmetry of HSC division. PLoS Biol. 2018;16:e2003389.
Pham K, Sacirbegovic F, Russell SM. Polarized cells, polarized views: asymmetric cell division in hematopoietic cells. Front Immunol. 2014;5:26.
Larsson J. Do HSCs divide asymmetrically? Blood J Am Soc Hematol. 2012;119:2431–2.
Loeffler D, Schneiter F, Schroeder T. Pitfalls and requirements in quantifying asymmetric mitotic segregation. Ann N. Y Acad Sci. 2020;1466:73–82.
Hoppe PS, Schwarzfischer M, Loeffler D, Kokkaliaris KD, Hilsenbeck O, Moritz N, et al. Early myeloid lineage choice is not initiated by random PU. 1 to GATA1 protein ratios. Nature. 2016;535:299–302.
Loeffler D, Wang W, Hopf A, Hilsenbeck O, Bourgine PE, Rudolf F, et al. Mouse and human HSPC immobilization in liquid culture by CD43-or CD44-antibody coating. Blood J Am Soc Hematol. 2018;131:1425–9.
Hilsenbeck O, Schwarzfischer M, Loeffler D, Dimopoulos S, Hastreiter S, Marr C, et al. fastER: a user-friendly tool for ultrafast and robust cell segmentation in large-scale microscopy. Bioinformatics. 2017;33:2020–8.
Peng T, Thorn K, Schroeder T, Wang L, Theis FJ, Marr C, et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat Commun. 2017;8:14836.
Dong S, Wang Q, Kao Y-R, Diaz A, Tasset I, Kaushik S, et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature. 2021;591:117–23.
García-Prat L, Kaufmann KB, Schneiter F, Voisin V, Murison A, Chen J, et al. TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate. Cell Stem Cell. 2021;28:1838–50.e10.
Nunes J, Loeffler D. Asymmetric cell division of hematopoietic stem cells: recent advances, emerging concepts, and future perspectives. Front Hematol. 2024;3:1373554.
Ting SB, Deneault E, Hope K, Cellot S, Chagraoui J, Mayotte N, et al. Asymmetric segregation and self-renewal of hematopoietic stem and progenitor cells with endocytic Ap2a2. Blood J Am Soc Hematol. 2012;119:2510–22.
Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, et al. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–8.
Sunchu B, Cabernard C. Principles and mechanisms of asymmetric cell division. Development. 2020;147:dev167650.
Hinge A, He J, Bartram J, Javier J, Xu J, Fjellman E, et al. Asymmetrically segregated mitochondria provide cellular memory of hematopoietic stem cell replicative history and drive HSC attrition. Cell Stem Cell. 2020;26:420–30.e6.
Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell. 2019;24:405–18.e7.
Görgens A, Ludwig A-K, Möllmann M, Krawczyk A, Dürig J, Hanenberg H, et al. Multipotent hematopoietic progenitors divide asymmetrically to create progenitors of the lymphomyeloid and erythromyeloid lineages. Stem Cell Rep. 2014;3:1058–72.
Loeffler D, Wehling A, Schneiter F, Zhang Y, Müller-Bötticher N, Hoppe PS, et al. Publisher Correction: Asymmetric lysosome inheritance predicts activation of haematopoietic stem cells. Nature. 2019;573:E5–E.
Ugale A, Shunmugam D, Pimpale LG, Rebhan E, Baccarini M Signaling proteins in HSC fate determination are unequally segregated during asymmetric cell division. J Cell Biol. 2024;223.
Zhou H, Li I, Bramlett CS, Wang B, Hao J, Yen DP, et al. Label-free metabolic optical biomarkers track stem cell fate transition in real time. Sci Adv. 2024;10:eadi6770.
Girotra M, Trachsel V, Roch A, Lutolf MP. In vivo pre-instructed HSCs robustly execute asymmetric cell divisions in vitro. Int J Mol Sci. 2020;21:8225.
Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science. 2016;354:1156–60.
Ellenbroek SI, Iden S, Collard JG, editors. Cell polarity proteins and cancer. Seminars in cancer biology; Elsevier (2012).
Bajaj J, Zimdahl B, Reya T. Fearful symmetry: subversion of asymmetric division in cancer development and progression. Cancer Res. 2015;75:792–7.
Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–8.
Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–54.
Mizukawa B, O’Brien E, Moreira DC, Wunderlich M, Hochstetler CL, Duan X, et al. The cell polarity determinant CDC42 controls division symmetry to block leukemia cell differentiation. Blood J Am Soc Hematol. 2017;130:1336–46.
Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–68.
Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–95.
Eifert T, Hsu C-J, Becker AL, Graessle S, Horne A, Bemmann F, et al. Cell fate determinant Llgl1 is required for propagation of acute myeloid leukemia. Leukemia. 2023;37:2027–35.
Prebet T, Lhoumeau A-C, Arnoulet C, Aulas A, Marchetto S, Audebert S, et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood J Am Soc Hematol. 2010;116:2315–23.
Jiang D, He Y, Mo Q, Liu E, Li X, Huang L, et al. PRICKLE1, a Wnt/PCP signaling component, is overexpressed and associated with inferior prognosis in acute myeloid leukemia. J Transl Med. 2021;19:211.
Zimdahl B, Ito T, Blevins A, Bajaj J, Konuma T, Weeks J, et al. Lis1 regulates asymmetric division in hematopoietic stem cells and in leukemia. Nat Genet. 2014;46:245–52.
Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–91.
Gur-Cohen S, Itkin T, Chakrabarty S, Graf C, Kollet O, Ludin A, et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med. 2015;21:1307–17.
Schuster T, Amoah A, Vollmer A, Marka G, Niemann J, Saçma M, et al. Quantitative determination of the spatial distribution of components in single cells with CellDetail. Nat Commun. 2024;15:10250.
Gong Y, Varnau R, Wallner ES, Acharya R, Bergmann DC, Cheung LS. Quantitative and dynamic cell polarity tracking in plant cells. N. Phytol. 2021;230:867–77.
Kaucká M, Plevová K, Pavlová Š, Janovská P, Mishra A, Verner J, et al. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res. 2013;73:1491–501.
Montserrat-Vazquez S, Ali NJ, Matteini F, Lozano J, Zhaowei T, Mejia-Ramirez E, et al. Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice. npj Regen Med. 2022;7:78.
Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–22.
Peglion F, Etienne-Manneville S. Cell polarity changes in cancer initiation and progression. J Cell Biol. 2023;223:e202308069.
Lang CF, Munro E. The PAR proteins: from molecular circuits to dynamic self-stabilizing cell polarity. Development. 2017;144:3405–16.
Funding
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute (NHLBI) of the National Institutes of Health (NIH) under Award Number R01HL174644. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was received from Alex’s Lemonade Stand Foundation and RUNX1 Research Program (Grant # 22-27080) and St. Jude Comprehensive Cancer Center Developmental Funds Grant (#PROJ-0003066 720202211).
Author information
Authors and Affiliations
Contributions
D.L. and S.B. wrote, edited, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bera, S., Loeffler, D. Cell polarity: cell type-specific regulators, common pathways, and polarized vesicle transport. Leukemia 39, 1558–1570 (2025). https://doi.org/10.1038/s41375-025-02601-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02601-x
This article is cited by
-
Macrophage polarization in hematologic cancers: mechanisms and therapeutic strategies
Blood Research (2026)
-
Haematopoietic ageing in health and lifespan
Nature Cell Biology (2025)