Abstract
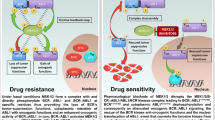
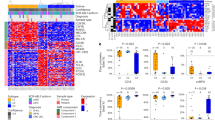
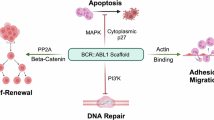
BCR::ABL1 oncofusion protein drives Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ ALL), making it a critical therapeutic target. Tyrosine kinase inhibitors (TKIs) targeting BCR::ABL1 have revolutionized the treatment of Ph+ ALL patients. However, mutations in the kinase domain of BCR::ABL1 commonly impair the sensitivity to TKIs, resulting in drug resistance and poor prognosis in Ph+ ALL. Here we report that heat stress selectively destabilizes BCR::ABL1 and its common drug-resistant mutants without affecting the native BCR and ABL proteins through inducing liquid-to-solid phase transition. Mechanistic studies revealed that heat stress facilitated recruitment of BCR::ABL1 signaling components (e.g., SHIP2, Sts1, PI3K-p85α and Shc) in a kinase activity dependent manner and stimulated BCR::ABL1 oligomerization through its coiled-coil domain, resulting in formation of a large, thermally unstable signaling complex. This process triggers non-canonical K27-linked ubiquitination mediated by c-Cbl E3 ubiquitin ligase, ultimately leading to BCR::ABL1 degradation via the ubiquitin-proteasome pathway. Functionally, heat stress effectively suppressed proliferation of BCR::ABL1-driven leukemia cells, including drug resistant mutants in vitro and decreased tumor burden in vivo. Our findings established that thermal-based therapy as a novel strategy to selectively target and degrade both unmutated and drug-resistant BCR::ABL1 oncoproteins, offering a promising adjuvant approach to overcome TKI resistance in Ph+ ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw sequence data have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2024), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA011771) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human. All other data reported in this paper will be shared by the lead contact upon request.
References
Foà R, Chiaretti S. Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2022;386:2399–411.
Mancini M, Scappaticci D, Cimino G, Nanni M, Derme V, Elia L, et al. A comprehensive genetic classification of adult acute lymphoblastic leukemia (ALL): analysis of the GIMEMA 0496 protocol. Blood. 2005;105:3434–41.
Burmeister T, Schwartz S, Bartram CR, Gökbuget N, Hoelzer D, Thiel E, et al. Patients’ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112:918–9.
Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–801.
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–86.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Goldstone, ECOG, MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–7.
Park HS. Current treatment strategies for Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Blood Res. 2020;55:S32–S36.
Mathisen MS, O’Brien S, Thomas D, Cortes J, Kantarjian H, Ravandi F. Role of tyrosine kinase inhibitors in the management of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2011;6:187–94.
Chiaretti S, Ansuinelli M, Vitale A, Elia L, Matarazzo M, Piciocchi A, et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica. 2021;106:1828–38.
Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–25.
Pfeifer H, Wassmann B, Pavlova A, Wunderle L, Oldenburg J, Binckebanck A, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2007;110:727–34.
Huang H, Weng H, Dong B, Zhao P, Zhou H, Qu L. Oridonin triggers chaperon-mediated proteasomal degradation of BCR-ABL in leukemia. Sci Rep. 2017;7:41525.
Lu Z, Jin Y, Qiu L, Lai Y, Pan J. Celastrol, a novel HSP90 inhibitor, depletes Bcr-Abl and induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation. Cancer Lett. 2010;290:182–91.
Shibata N, Ohoka N, Tsuji G, Demizu Y, Miyawaza K, Ui-Tei K, et al. Deubiquitylase USP25 prevents degradation of BCR-ABL protein and ensures proliferation of Ph-positive leukemia cells. Oncogene. 2020;39:3867–78.
Jiang S, Wang X, He Y, Huang H, Cao B, Zhang Z, et al. Suppression of USP7 induces BCR-ABL degradation and chronic myelogenous leukemia cell apoptosis. Cell Death Dis. 2021;12:456.
Yang Y, Gao H, Sun X, Sun Y, Qiu Y, Weng Q, et al. Global PROTAC toolbox for degrading BCR-ABL overcomes drug-resistant mutants and adverse effects. J Med Chem. 2020;63:8567–83.
Liu H, Ding X, Liu L, Mi Q, Zhao Q, Shao Y, et al. Discovery of novel BCR-ABL PROTACs based on the cereblon E3 ligase design, synthesis, and biological evaluation. Eur J Med Chem. 2021;223:113645.
Wang J, Wang Y, Yang F, Luo Q, Hou Z, Xing Y, et al. A novel lysosome targeting chimera for targeted protein degradation via split-and-mix strategy. ACS Chem Biol. 2024;19:1161–8.
Zhang J, Ma C, Yu Y, Liu C, Fang L, Rao H. Single amino acid-based PROTACs trigger degradation of the oncogenic kinase BCR-ABL in chronic myeloid leukemia (CML). J Biol Chem. 2023;299:104994.
Ma B, Feng H, Feng C, Liu Y, Zhang H, Wang J, et al. Kill two birds with one stone: a multifunctional dual-targeting protein drug to overcome imatinib resistance in Philadelphia chromosome-positive leukemia. Adv Sci. 2022;9:e2104850.
Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366–83.
Daccache A, Kizhakekuttu T, Siebert J, Veeder M. Hematologic and cytogenetic spontaneous remission in acute monocytic leukemia (FAB M5b) with trisomy 8. J Clin Oncol. 2007;25:344–6.
Hobohm U. Fever therapy revisited. Brit J Cancer. 2005;92:421.
Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, et al. Phase I clinical trial of mixed bacterial vaccine (Coley’s toxins) in patients with NY-ESO-1 expressing cancers: immunological effects and clinical activity. Clin Cancer Res. 2012;18:5449–59.
Maimaitiyiming Y, Wang QQ, Yang C, Ogra Y, Lou Y, Smith CA, et al. Hyperthermia selectively destabilizes oncogenic fusion proteins. Blood Cancer Discov. 2021;2:388–401.
Wang QQ, Hussain L, Yu PH, Yang C, Zhu CY, Ma YF, et al. Hyperthermia promotes degradation of the acute promyelocytic leukemia driver oncoprotein ZBTB16/RARα. Acta Pharm Sin. 2023;44:822–31.
Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354.
Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44.
Wu J, Meng F, Lu H, Kong L, Bornmann W, Peng Z, et al. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood. 2008;111:3821–9.
Brehme M, Hantschel O, Colinge J, Kaupe I, Planyavsky M, Köcher T, et al. Charting the molecular network of the drug target Bcr-Abl. Proc Natl Acad Sci USA. 2009;106:7414–9.
Gregor T, Bosakova MK, Nita A, Abraham SP, Fafilek B, Cernohorsky NH, et al. Elucidation of protein interactions necessary for the maintenance of the BCR-ABL signaling complex. Cell Mol Life Sci. 2020;77:3885–903.
Amarante-Mendes GP, Rana A, Datoguia TS, Hamerschlak N, Brumatti G. BCR-ABL1 tyrosine kinase complex signaling transduction: challenges to overcome resistance in chronic myeloid leukemia. Pharmaceutics. 2022;14:215.
Dixon AS, Pendley SS, Bruno BJ, Woessner DW, Shimpi AA, Cheatham TE, et al. Disruption of Bcr-Abl coiled coil oligomerization by design. J Biol Chem. 2011;286:27751–60.
Burslem GM. The chemical biology of ubiquitin. Biochim Biophys Acta Gen Subj. 2022;1866:130079.
Damgaard RB. The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021;28:423–6.
Kong L, Sui C, Chen T, Zhang L, Zhao W, Zheng Y, et al. The ubiquitin E3 ligase TRIM10 promotes STING aggregation and activation in the Golgi apparatus. Cell Rep. 2023;42:112306.
Yang Q, Zhao J, Chen D, Wang Y. E3 ubiquitin ligases: styles, structures and functions. Mol Biomed. 2021;2:23.
Wang D, Zhang Y, Xu X, Wu J, Peng Y, Li J, et al. YAP promotes the activation of NLRP3 inflammasome via blocking K27-linked polyubiquitination of NLRP3. Nat Commun. 2021;12:2674.
Shearer RF, Typas D, Coscia F, Schovsbo S, Kruse T, Mund A, et al. K27-linked ubiquitylation promotes p97 substrate processing and is essential for cell proliferation. EMBO J. 2022;41:e110145.
Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol. 2018;1:100.
Sun Y, Zhao X, Ding N, Gao H, Wu Y, Yang Y, et al. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018;28:779–81.
Wu RH, Zhu CY, Yu PH, Ma Y, Hussain L, Naranmandura H, et al. The landscape of novel strategies for acute myeloid leukemia treatment: therapeutic trends, challenges, and future directions. Toxicol Appl Pharm. 2023;473:116585.
Sun X, Rao Y. PROTACs as potential therapeutic agents for cancer drug resistance. Biochemistry. 2020;59:240–9.
Pan YL, Zeng SX, Hao RR, Liang MH, Shen ZR, Huang WH. The progress of small-molecules and degraders against BCR-ABL for the treatment of CML. Eur J Med Chem. 2022;238:114442.
Miettinen TP, Peltier J, Härtlova A, Gierliński M, Jansen VM, Trost M. Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J. 2018;37:e98359.
Savitski MM, Reinhard FB, Franken H, Werner T, Savitski MF, Eberhard D, et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science. 2014;346:1255784.
Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–49.
Acknowledgements
This manuscript was funded by National Natural Science Foundation of China (No. 82170143, 82370191, 82200160; 31970706).
Author information
Authors and Affiliations
Contributions
Conception: CY, MB, HN; Data curation: CY, YYK, CYZ, YQL, ZYZ; Methodology: CY, YYK, CYZ, YFM, PHY, YO, NS; Resources: CY, YFM, YO, NS, HN; Investigation: CY, YYK, CYZ, PHY, TY, MB, HN; Visualization: YYK, CYZ, TY, YQL, ZYZ; Funding acquisition: CY, MB, HN; Project administration: CY, MB, HN; Supervision: MB, HN; Writing–original draft: CY, YYK, CYZ, MB, HN; Writing–review and editing: CY, YYK, CYZ, YO, NS, MB, HN.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Kang, YY., Zhu, CY. et al. Heat stress targets and degrades BCR::ABL1 oncoproteins to overcome drug-resistance in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 39, 2140–2151 (2025). https://doi.org/10.1038/s41375-025-02709-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02709-0