Abstract
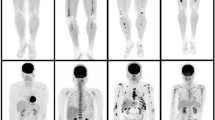
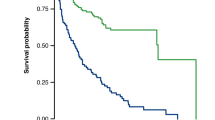
The co-primary aims of this study were to assess the diagnostic and prognostic performance of Whole-body-2-[18F]-fluorodeoxyglucose-positron emission tomography coupled with MRI (WB-2-[18F]FDG-PET/MRI) imaging in the smoldering multiple myeloma (SMM) workup for detection of MM-related medullary or extra-medullary disease and for the prediction of progression-free survival (PFS) defined as time to progression to symptomatic MM requiring therapy. A total of 116 patients with SMM (without CRAB or SLiM criteria before imaging) were prospectively included in the study and underwent full multi-parametric WB-2-[18F]FDG-PET/MRI imaging. PET detected at least one FL > 5 mm in 9% of patients compared to 20% on MRI (p = 0.02). PET detected diffuse bone marrow involvement (BMI) in 20% of patients and MRI in 53% (p < 10−3). A total of 98 patients with true SMM not requiring treatment were then followed up. The presence of diffuse BMI on MRI was the strongest adverse prognostic parameter for PFS (univariate: hazard ratio (HR), 6.12; p < 10−2. Multivariate : HR, 4.16; p = 0.03) and could be proposed as a new high-risk biomarker for progression to symptomatic MM. Dynamic contrast-enhanced (DCE)-MRI based increased peak enhancement intensity (PEI) and maximum intensity time ratio (MITR) values were other strong adverse prognostic factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The anonymized datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125:3069–75.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015;33:657–64.
Hillengass J, Zechmann C, Bäuerle T, Wagner-Gund B, Heiss C, Benner A, et al. Dynamic contrast-enhanced magnetic resonance imaging identifies a subgroup of patients with asymptomatic monoclonal plasma cell disease and pathologic microcirculation. Clin Cancer Res. 2009;15:3118–25.
Bhutani M, Turkbey B, Tan E, Kemp TJ, Pinto LA, Berg AR, et al. Bone marrow angiogenesis in myeloma and its precursor disease: a prospective clinical trial. Leukemia. 2014;28:413–6.
Merz M, Moehler TM, Ritsch J, Bäuerle T, Zechmann CM, Wagner B, et al. Prognostic significance of increased bone marrow microcirculation in newly diagnosed multiple myeloma: results of a prospective DCE-MRI study. Eur Radio. 2016;26:1404–11.
Hillengass J, Ritsch J, Merz M, Wagner B, Kunz C, Hielscher T, et al. Increased microcirculation detected by dynamic contrast-enhanced magnetic resonance imaging is of prognostic significance in asymptomatic myeloma. Br J Haematol. 2016;174:127–35.
Zamagni E, Nanni C, Gay F, Pezzi A, Patriarca F, Bellò M, et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia. 2016;30:417–22.
Siontis B, Kumar S, Dispenzieri A, Drake MT, Lacy MQ, Buadi F, et al. Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy. Blood Cancer J. 2015;5:e364.
Benaniba L, Tessoulin B, Trudel S, Pellat-Deceunynck C, Amiot M, Minvielle S, et al. The MYRACLE protocol study: a multicentric observational prospective cohort study of patients with multiple myeloma. BMC Cancer. 2019;19:855.
Messiou C, Hillengass J, Delorme S, Lecouvet F, Moulopoulos L, Collins DJ, et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS). Radiology. 2019;291:5–13.
Jamet B, Necib H, Carlier T, Frampas E, Bazin J, Desfontis PH, et al. DCE-MRI to distinguish all monoclonal plasma cell disease stages and correlation with diffusion-weighted MRI/PET-based biomarkers in a hybrid simultaneous whole body-2-[18F]FDG-PET/MRI imaging approach. Cancer Imaging. 2024;24:93.
Dutoit J, Vanderkerken M, Verstraete Koenraad L. Value of whole body MRI and dynamic contrast enhanced MRI in the diagnosis, follow-up and evaluation of disease activity and extent in multiple myeloma. Eur J Radio. 2013;82:1444–52.
Terpos E, Matsaridis D, Koutoulidis V, Zagouri F, Christoulas D, Fontara S, et al. Dynamic contrast-enhanced magnetic resonance imaging parameters correlate with advanced revised-ISS and angiopoietin-1/angiopoietin-2 ratio in patients with multiple myeloma. Ann Hematol. 2017;96:1707–14.
Messiou C, Collins DJ, Morgan VA, Desouza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radio. 2011;21:1713–8.
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magnetic Reson Imaging. 2012;30:1323–41.
Messiou C, Porta N, Sharma B, Levine D, Koh DM, Boyd K, et al. Prospective evaluation of whole-body MRI versus FDG PET/CT for lesion detection in participants with myeloma. Radiol Imaging Cancer. 2021;3:e210048.
Hillengass J, Usmani S, Rajkumar SV, Durie B, Mateos M-V, Lonialet S, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302–12.
Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–5.
Paschali A, Panagiotidis E, Triantafyllou T, Palaska V, Tsirou K. Verrouet E al. A proposed index of diffuse bone marrow [18F]-FDG uptake and PET skeletal patterns correlate with myeloma prognostic markers, plasma cell morphology, and response to therapy. Eur J Nucl Med Mol Imaging. 2021;48:1487–97.
Hillengass J, Fechtner K, Weber MA, Bauerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606–10.
Kastritis E, Terpos E, Moulopoulos L, Spyropoulou-Vlachou M, Kanellias N, Eleftherakis-Papaiakovou E, et al. Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia. 2013;27:947–53.
Kastritis E, Moulopoulos LA, Terpos E, Koutoulidis V, Dimopoulos MA. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia. 2014;28:2402–3.
Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991;15:621–8.
Zhang J, Chen Y, Zhang Y, Zhang E, Yu HJ, Yuan H, et al. Diagnosis of spinal lesions using perfusion parameters measured by DCE-MRI and metabolism parameters measured by PET/CT. Eur Spine J. 2020;29:1061–70.
Jamet B, Morvan L, Nanni C, Michaud AV, Bailly C, Chauvie S, et al. Random survival forest to predict transplant-eligible newly diagnosed multiple myeloma outcome including FDG-PET radiomics: a combined analysis of two independent prospective European trials. Eur J Nucl Med Mol Imaging. 2021;48:1005–15.
Lakshman A, Rajkumar SV, Buadi FK, Binder M, Gertz MA, Lacy MQ, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood. Cancer J. 2018;8:59.
Acknowledgements
This work has been supported in part by the French National Research Agency (ANR) “France 2030 investment plan” under the references I-SITE NExT (ANR-16-IDEX-0007) and Labex DHOLMEN, by financial support from the Pays de la Loire Region, by a grant from INCa-DGOS-INSERM/ITMO Cancer_18011 (SIRIC ILIAD) and by Siemens Healthineers, the industrial partner of the NExT research industrial chair IMRAM.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: BJ, FKB. Provision of study patients: CT, NB, PM. BJ, CT, HN, EF, HS, CF, NB, TC, AM, NL, CBM, PM and FKB contributed to the study execution, the collection and assembly of data, the data analysis and interpretation and the manuscript writing. BJ, CT, HN, EF, HS, CF, NB, TC, AM, NL, CBM, PM and FKB provided a full review of the manuscript and are fully responsible for all content and editorial decisions, were involved in all stages of manuscript development, and have approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jamet, B., Touzeau, C., Necib, H. et al. Simultaneous whole-body multi-parametric 2-[18F]FDG-PET/MRI in smoldering multiple myeloma assessment: diagnostic and prognostic impact. Leukemia 39, 2516–2522 (2025). https://doi.org/10.1038/s41375-025-02717-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02717-0