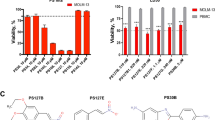
Abstract
Amino acid homeostasis is critical for leukemic cell survival, with the mTOR pathway playing a central role in sensing and responding to nutrient availability. DEPTOR, a component and negative regulator of mTOR complexes, has been extensively studied in solid tumors and multiple myeloma, but its role in acute myeloid leukemia (AML) remains unclear. Here, we identify DEPTOR as a key regulator of leukemia progression through its interaction with KIF11. DEPTOR expression is transcriptionally induced by ATF4 and post-transcriptionally stabilized by MSI2, which binds to DEPTOR mRNA and prevents its degradation. DEPTOR is highly expressed in leukemia stem cells (LSCs) and is associated with poor clinical outcomes. Functionally, DEPTOR loss impairs leukemogenesis in both AML and blast phase chronic myeloid leukemia (bpCML) models, without affecting normal hematopoietic stem cells. Mechanistically, DEPTOR stabilizes KIF11 by preventing its ubiquitination and proteasomal degradation, thereby ensuring proper mTORC1 localization and metabolic adaptation during nutrient stress. Collectively, our findings establish the MSI2/DEPTOR/KIF11 axis as a critical driver of leukemogenesis and a promising therapeutic target for aggressive myeloid leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the findings of this study are provided within the article. Additional information related to this research is available upon request from the corresponding author.
References
Sembill S, Ampatzidou M, Chaudhury S, Dworzak M, Kalwak K, Karow A, et al. Management of children and adolescents with chronic myeloid leukemia in blast phase: International pediatric CML expert panel recommendations. Leukemia. 2023;37:505–17.
Liu J, Jiang P, Lu Z, Yu Z, Qian P. Decoding leukemia at the single-cell level: clonal architecture, classification, microenvironment, and drug resistance. Experimental Hematol Oncol. 2024;13:12.
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
Bhingarkar A, Vangapandu HV, Rathod S, Hoshitsuki K, Fernandez CA. Amino acid metabolic vulnerabilities in acute and chronic myeloid leukemias. Front Oncol. 2021;11:694526.
Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24:1859–66.
Grønningsæter IS, Fredly HK, Gjertsen BT, Hatfield KJ, Bruserud Ø. Systemic metabolomic profiling of acute myeloid leukemia patients before and during disease-stabilizing treatment based on all-trans retinoic acid, valproic acid, and low-dose chemotherapy. Cells. 2019;8:1229.
Butler M, van der Meer LT, van Leeuwen FN. Amino acid depletion therapies: starving cancer cells to death. Trends Endocrinol Metab. 2021;32:367–81.
Fulton TL, Mirth CK, Piper MDW. Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Open Biol. 2022;12:220319.
Timosenko E, Ghadbane H, Silk JD, Shepherd D, Gileadi U, Howson LJ, et al. Nutritional stress induced by tryptophan-degrading enzymes results in ATF4-dependent reprogramming of the amino acid transporter profile in tumor cells. Cancer Res. 2016;76:6193–204.
Poulain L, Sujobert P, Zylbersztejn F, Barreau S, Stuani L, Lambert M, et al. High mTORC1 activity drives glycolysis addiction and sensitivity to G6PD inhibition in acute myeloid leukemia cells. Leukemia. 2017;31:2326–35.
Catena V, Fanciulli M. Deptor: not only a mTOR inhibitor. J Exp Clin Cancer Res. 2017;36:12.
Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86.
Wang J, Chen J, Qiu D, Zeng Z. Regulatory role of DEPTOR‑mediated cellular autophagy and mitochondrial reactive oxygen species in angiogenesis in multiple myeloma. Int J Mol Med. 2021;47:643–58.
Li N, Yousefi M, Nakauka-Ddamba A, Li F, Vandivier L, Parada K, et al. The Msi family of RNA-binding proteins function redundantly as intestinal oncoproteins. Cell Rep. 2015;13:2440–55.
Huang L, Sun J, Ma Y, Chen H, Tian C, Dong M. MSI2 regulates NLK-mediated EMT and PI3K/AKT/mTOR pathway to promote pancreatic cancer progression. Cancer Cell Int. 2024;24:273.
Kwon HY, Bajaj J, Ito T, Blevins A, Konuma T, Weeks J, et al. Tetraspanin 3 Is required for the development and propagation of acute myelogenous leukemia. Cell Stem Cell. 2015;17:152–64.
Li F, Han Y, Chen R, Jiang Y, Chen C, Wang X, et al. MicroRNA-143 acts as a tumor suppressor through Musashi-2/DLL1/Notch1 and Musashi-2/Snail1/MMPs axes in acute myeloid leukemia. J Transl Med. 2023;21:309.
Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–8.
Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med. 2010;16:903–8.
Doyle A, McGarry MP, Lee NA, Lee JJ. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012;21:327–49.
Hu W, Xiong H, Ru Z, Zhao Y, Zhou Y, Xie K, et al. Extracellular vesicles-released parathyroid hormone-related protein from Lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 2021;12:134.
Cunningham A, Erdem A, Alshamleh I, Geugien M, Pruis M, Pereira-Martins DA, et al. Dietary methionine starvation impairs acute myeloid leukemia progression. Blood J Am Soc Hematol. 2022;140:2037–52.
Sun J, Sheng W, Ma Y, Dong M. Potential role of musashi-2 RNA-binding protein in cancer EMT. Onco Targets Ther. 2021;14:1969–80.
Paz I, Kosti I, Ares M Jr, Cline M, Mandel-Gutfreund Y. RBPmap: a web server for mapping binding sites of RNA-binding proteins. Nucleic Acids Res. 2014;42:W361–7.
Rasmussen BB, Adams CM. ATF4 is a fundamental regulator of nutrient sensing and protein turnover. J Nutr. 2020;150:979–80.
Luo Y, Hitz BC, Gabdank I, Hilton JA, Kagda MS, Lam B, et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res. 2020;48:D882–d9.
Hitz BC, Lee J-W, Jolanki O, Kagda MS, Graham K, Sud P, et al. The ENCODE uniform analysis pipelines. bioRxiv. 2023:2023.04.04.535623.
Jin H-O, Hong S-E, Kim J-Y, Jang S-K, Park I-C. Amino acid deprivation induces AKT activation by inducing GCN2/ATF4/REDD1 axis. Cell Death Dis. 2021;12:1127.
Demetriades C, Doumpas N, Teleman Aurelio A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–99.
Takahara T, Amemiya Y, Sugiyama R, Maki M, Shibata H. Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes. J Biomed Sci. 2020;27:87.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102.
Sari IN, Yang Y-G, Wijaya YT, Jun N, Lee S, Kim KS, et al. AMD1 is required for the maintenance of leukemic stem cells and promotes chronic myeloid leukemic growth. Oncogene. 2021;40:603–17.
Zhang S, You X, Zheng Y, Shen Y, Xiong X, Sun Y. The UBE2C/CDH1/DEPTOR axis is an oncogene and tumor suppressor cascade in lung cancer cells. J Clin Investig. 2023;133:e162434.
Iqbal MJ, Kabeer A, Abbas Z, Siddiqui HA, Calina D, Sharifi-Rad J, et al. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun Signal. 2024;22:7.
Carter BZ, Mak DH, Shi Y, Schober WD, Wang R-Y, Konopleva M, et al. Regulation and targeting of Eg5, a mitotic motor protein in blast crisis CML: overcoming imatinib resistance. Cell Cycle. 2006;5:2223–9.
Kantarjian HM, Padmanabhan S, Stock W, Tallman MS, Curt GA, Li J, et al. Phase I/II multicenter study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD4877 in patients with refractory acute myeloid leukemia. Investig N Drugs. 2012;30:1107–15.
Eguren M, Álvarez-Fernández M, García F, López-Contreras AJ, Fujimitsu K, Yaguchi H, et al. A synthetic lethal interaction between APC/C and topoisomerase poisons uncovered by proteomic screens. Cell Rep. 2014;6:670–83.
Liu Y, Wang Y, Du Z, Yan X, Zheng P, Liu Y. Fbxo30 regulates mammopoiesis by targeting the bipolar mitotic kinesin Eg5. Cell Rep. 2016;15:1111–22.
Jang YG, Choi Y, Jun K, Chung J. Mislocalization of TORC1 to lysosomes caused by KIF11 inhibition leads to aberrant TORC1 activity. Mol Cells. 2020;43:705–17.
Shahin R, Aljamal S. Kinesin spindle protein inhibitors in cancer: from high throughput screening to novel therapeutic strategies. Future Sci OA. 2022;8:Fso778.
Zhou Y, Xu M-F, Chen J, Zhang J-L, Wang X-Y, Huang M-H, et al. Loss-of-function of kinesin-5 KIF11 causes microcephaly, chorioretinopathy, and developmental disorders through chromosome instability and cell cycle arrest. Experimental Cell Res. 2024;436:113975.
Villar VH, Nguyen TL, Delcroix V, Terés S, Bouchecareilh M, Salin B, et al. mTORC1 inhibition in cancer cells protects from glutaminolysis-mediated apoptosis during nutrient limitation. Nat Commun. 2017;8:14124.
Hoshii T, Tadokoro Y, Naka K, Ooshio T, Muraguchi T, Sugiyama N, et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Investig. 2012;122:2114–29.
Fang Y, Yang Y, Hua C, Xu S, Zhou M, Guo H, et al. Rictor has a pivotal role in maintaining quiescence as well as stemness of leukemia stem cells in MLL-driven leukemia. Leukemia. 2017;31:414–22.
Lechman Eric R, Gentner B, Ng Stanley WK, Schoof Erwin M, van Galen P, Kennedy James A, et al. miR-126 regulates distinct self-renewal outcomes in normal and malignant hematopoietic stem cells. Cancer Cell. 2016;29:214–28.
Wu F, Chen Z, Liu J, Hou Y. The Akt–mTOR network at the interface of hematopoietic stem cell homeostasis. Experimental Hematol. 2021;103:15–23.
Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korean Government (MSIT) (NRF-2023R1A2C1003952) and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (RS-2024-00437643).
Author information
Authors and Affiliations
Contributions
TS and JAM planned, designed, and performed the majority of experiments and helped write the manuscript. NM, LMW, NJ, and INS performed mechanism studies, and VGO and DK analyzed patient-derived data. HYK planned and guided the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Declaration of Generative AI and AI-assisted technologies in the writing process: During the preparation of this work, the author(s) used ChatGPT in order to improve readability and language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Ethical approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All patients were provided written informed consent to participate in studies and for the research use of their specimens in agreement with the Declaration of Helsinki. Animal protocols were reviewed and approved by Soonchunhyang University Institutional Animal Care and Use Committee.
Consent for publication
This manuscript has not been published previously and is not under consideration for publication elsewhere.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Setiawan, T., Muhammad, J.A., Marcellina, N. et al. Regulation of metabolic adaptation and leukemia progression by MUSASHI2-DEPTOR-KIF11 axis. Leukemia (2025). https://doi.org/10.1038/s41375-025-02768-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41375-025-02768-3