Abstract
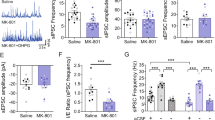
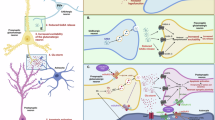
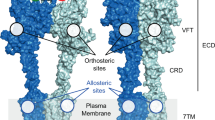
Recent clinical and preclinical studies suggest that selective activators of the M4 muscarinic acetylcholine receptor have potential as a novel treatment for schizophrenia. M4 activation inhibits striatal dopamine release by mobilizing endocannabinoids, providing a mechanism for local effects on dopamine signaling in the striatum but not in extrastriatal areas. G protein-coupled receptors (GPCRs) typically induce endocannabinoid release through activation of Gαq/11-type G proteins whereas M4 transduction occurs through Gαi/o-type G proteins. We now report that the ability of M4 to inhibit dopamine release and induce antipsychotic-like effects in animal models is dependent on co-activation of the Gαq/11-coupled mGlu1 subtype of metabotropic glutamate (mGlu) receptor. This is especially interesting in light of recent findings that multiple loss of function single nucleotide polymorphisms (SNPs) in the human gene encoding mGlu1 (GRM1) are associated with schizophrenia, and points to GRM1/mGlu1 as a gene within the “druggable genome” that could be targeted for the treatment of schizophrenia. Herein, we report that potentiation of mGlu1 signaling following thalamo-striatal stimulation is sufficient to inhibit striatal dopamine release, and that a novel mGlu1 positive allosteric modulator (PAM) exerts robust antipsychotic-like effects through an endocannabinoid-dependent mechanism. However, unlike M4, mGlu1 does not directly inhibit dopamine D1 receptor signaling and does not reduce motivational responding. Taken together, these findings highlight a novel mechanism of cross talk between mGlu1 and M4 and demonstrate that highly selective mGlu1 PAMs may provide a novel strategy for the treatment of positive symptoms associated with schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53:119–33.
Kaiser R, Tremblay PB, Klufmoller F, Roots I, Brockmoller J. Relationship between adverse effects of antipsychotic treatment and dopamine D(2) receptor polymorphisms in patients with schizophrenia. Mol Psychiatry. 2002;7:695–705.
Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–9.
Barak S, Weiner I. The M(1)/M(4) preferring agonist xanomeline reverses amphetamine-, MK801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. Int J Neuropsychopharmacol. 2011;14:1233–46.
Shannon HE, Rasmussen K, Bymaster FP, Hart JC, Peters SC, Swedberg MD, et al. Xanomeline, an M(1)/M(4) preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr Res. 2000;42:249–59.
Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, et al. The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther. 2001;299:782–92.
Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA. Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur J Pharmacol. 2009;603:147–9.
Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327:941–53.
Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–42.
Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, et al. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–93.
Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, et al. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 2016;91:1244–52.
Scarr E, Um JY, Cowie TF, Dean B. Cholinergic muscarinic M4 receptor gene polymorphisms: a potential risk factor and pharmacogenomic marker for schizophrenia. Schizophr Res. 2013;146:279–84.
Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47.
Nishi A, Shuto T. Potential for targeting dopamine/DARPP-32 signaling in neuropsychiatric and neurodegenerative disorders. Expert Opin Ther Targets. 2017;21:259–72.
Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry. 2017;81:31–42.
Moehle MS, Pancani T, Byun N, Yohn SE, Willison GH, Dickerson JW, et al. Cholinergic projections to the substantia nigra reticulata inhibit dopamine modulation of basal ganglia through the M4 muscarinic receptor. Neuron. 2017;96:1358–72.
Gyombolai P, Pap D, Turu G, Catt KJ, Bagdy G, Hunyady L. Regulation of endocannabinoid release by G proteins: a paracrine mechanism of G protein-coupled receptor action. Mol Cell Endocrinol. 2012;353:29–36.
Szekeres M, Nadasy GL, Turu G, Soltesz-Katona E, Benyo Z, Offermanns S, et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol Cell Endocrinol. 2015;403:46–56.
Testa CM, Standaert DG, Young AB, Penney JB Jr. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–18.
Kerner JA, Standaert DG, Penney JB Jr., Young AB, Landwehrmeyer GB. Expression of group one metabotropic glutamate receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Brain Res Mol Brain Res. 1997;48:259–69.
Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66.
Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2 + dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–68.
Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. Mol Cell Endocrinol. 2010;44:75–85.
Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322.
Cho HP, Garcia-Barrantes PM, Brogan JT, Hopkins CR, Niswender CM, Rodriguez AL, et al. Chemical modulation of mutant mGlu1 receptors derived from deleterious GRM1 mutations found in schizophrenics. ACS Chem Biol. 2014;9:2334–46.
Ayoub MA, Angelicheva D, Vile D, Chandler D, Morar B, Cavanaugh JA, et al. Deleterious GRM1 mutations in schizophrenia. PLoS ONE. 2012;7:e32849.
Covey DP, Juliano SA, Garris PA. Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS ONE. 2013;8:e60763.
Wong JM, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J Chromatogr A. 2016;1446:78–90.
Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. JoVE. 2015;96:e52434.
Valsamis B, Schmid S. Habituation and prepulse inhibition of acoustic startle in rodents. JoVE. 2011;55:e3446..
Wilson AN, Gratz OH. Using a progressive ratio schedule of reinforcement as an assessment tool to inform treatment. Behav Anal Pract. 2016;9:257–60.
Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–7.
Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–59.
Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–20.
Zhang H, Sulzer D, Glutamate spillover in the striatum depresses dopaminergic transmission by activating group. I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–92.
Garcia-Barrantes PM, Cho HP, Starr TM, Blobaum AL, Niswender CM, Conn PJ, et al. Re-exploration of the mGlu(1) PAM Ro 07-11401 scaffold: Discovery of analogs with improved CNS penetration despite steep SAR. Bioorg Med Chem Lett. 2016;26:2289–92.
Garcia-Barrantes PM, Cho HP, Metts AM, Blobaum AL, Niswender CM, Conn PJ, et al. Lead optimization of the VU0486321 series of mGlu(1) PAMs. Part 2: SAR of alternative 3-methyl heterocycles and progress towards an in vivo tool. Bioorg Med Chem Lett. 2016;26:751–6.
Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, et al. Synaptically driven endocannabinoid release requires Ca2 + -assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–35.
Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–81.
Zheng T, Wilson CJ. Corticostriatal combinatorics: the implications of corticostriatal axonal arborizations. J Neurophysiol. 2002;87:1007–17.
Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–7.
Kosillo P, Zhang YF, Threlfell S, Cragg SJ. Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb Cortex. 2016;26:4160–9.
Morari M, Marti M, Sbrenna S, Fuxe K, Bianchi C, Beani L. Reciprocal dopamine-glutamate modulation of release in the basal ganglia. Neurochem Int. 1998;33:383–97.
van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–70.
Roffman JL, Tanner AS, Eryilmaz H, Rodriguez-Thompson A, Silverstein NJ, Ho NF, et al. Dopamine D1 signaling organizes network dynamics underlying working memory. Sci Adv. 2016;2:e1501672.
Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, et al. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–12.
Chiken S, Sato A, Ohta C, Kurokawa M, Arai S, Maeshima J, et al. Dopamine D1 receptor-mediated transmission maintains information flow through the cortico-striato-entopeduncular direct pathway to release movements. Cereb Cortex. 2015;25:4885–97.
Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, et al. The role of dopamine D1 receptor transmission in effort-related choice behavior: Effects of D1 agonists. Pharmacol Biochem Behav. 2015;135:217–26.
Cazorla M, Kang UJ, Kellendonk C. Balancing the basal ganglia circuitry: a possible new role for dopamine D2 receptors in health and disease. Mov Disord. 2015;30:895–903.
Avery MC, Krichmar JL. Improper activation of D1 and D2 receptors leads to excess noise in prefrontal cortex. Front Comput Neurosci. 2015;9:31.
Horga G, Cassidy CM, Xu X, Moore H, Slifstein M, Van Snellenberg JX, et al. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 2016;73:862–70.
Abi-Dargham A. Probing cortical dopamine function in schizophrenia: what can D1 receptors tell us? World Psychiatry. 2003;2:166–71.
Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40:1328–37.
Olarte-Sanchez CM, Valencia-Torres L, Cassaday HJ, Bradshaw CM, Szabadi E. Effects of SKF-83566 and haloperidol on performance on progressive ratio schedules maintained by sucrose and corn oil reinforcement: quantitative analysis using a new model derived from the Mathematical Principles of Reinforcement (MPR). Psychopharmacology. 2013;230:617–30.
Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8.
Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a Progressive Ratio task. Schizophr Res. 2016;170:198–204.
Ziauddeen H, Dibben C, Kipps C, Hodges JR, McKenna PJ. Negative schizophrenic symptoms and the frontal lobe syndrome: one and the same. Eur Arch Psychiatry Clin Neurosci. 2011;261:59–67.
Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, et al. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001;108:65–78.
Patel NH, Vyas NS, Puri BK, Nijran KS, Al-Nahhas A. Positron emission tomography in schizophrenia: a new perspective. J Nucl Med. 2010;51:511–20.
Farber NB, Newcomer JW, Olney JW. The glutamate synapse in neuropsychiatric disorders. Focus on schizophrenia and Alzheimer’s disease. Prog Brain Res. 1998;116:421–37.
Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends Mol Med. 2011;17:689–98.
Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012;213:267–95.
Rubio MD, Drummond JB, Meador-Woodruff JH. Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther. 2012;20:1–18.
Pietraszek M, Nagel J, Gravius A, Schafer D, Danysz W. The role of group I metabotropic glutamate receptors in schizophrenia. Amino Acids. 2007;32:173–8.
Mao L, Conquet F, Wang JQ. Augmented motor activity and reduced striatal preprodynorphin mRNA induction in response to acute amphetamine administration in metabotropic glutamate receptor 1 knockout mice. Neuroscience. 2001;106:303–12.
Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–69.
Brunelin J, Fecteau S, Suaud-Chagny MF. Abnormal striatal dopamine transmission in schizophrenia. Curr Med Chem. 2013;20:397–404.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9.
Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–3.
Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–24.
Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–9.
Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–6.
Salamone JD, Correa M, Yohn S, Lopez Cruz L, San Miguel N, Alatorre L. The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behav Process. 2016;127:3–17.
Salamone JD, Koychev I, Correa M, McGuire P. Neurobiological basis of motivational deficits in psychopathology. Eur Neuropsychopharmacol. 2015;25:1225–38.
Covey DP, Dantrassy HM, Zlebnik NE, Gildish I, Cheer JF. Compromised dopaminergic encoding of reward accompanying suppressed willingness to overcome high effort costs is a prominent prodromal characteristic of the Q175 mouse model of Huntington’s Disease. J Neurosci. 2016;36:4993–5002.
Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem. 1998;70:586A–92A.
Acknowledgements
We would like to thank Douglas Shaw and Ginger Milne for their invaluable assistance. This work was supported by funding from an NIH Institutional Training Grant (T32 MH065215-14) and Ruth L. Kirschstein National Research Service Award (F32MH113266) to SEY, a NARSAD Young Investigator Award to DJF, grants to PJC from National Institute of Mental Health (MH062646) and the National Institute of Neurological Disease and Stroke (NS031373), National Institute on Drug Abuse grants to JFC (DA022340 and DA042595) and a NARSA to DPC (DA041827). Research conducted by the Vanderbilt Neurochemistry Core is supported by the EKS NICHD of the NIH (U54HD083211). Data generated by the Neurochemistry Core is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions:
SEY and PJC conceived the studies and wrote the manuscript. SEY, DJF, DPC, MSM, CKJ, JFC, and MB designed experiments. SEY, DPC, MSM, JG, ALB, HPC, and MB conducted experiments and analyzed the data. CWL and PMBG provided pharmacological tools utilized in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CWL and PJC are inventors on patents that protect different classes of metabotropic glutamate allosteric modulators. CWL has been funded by the NIH, Johnson and Johnson, Bristol-Myers Squibb, AstraZeneca, Michael J. Fox Foundation, as well as Seaside Therapeutics. He has consulted for AbbVie and received compensation. PJC has been funded by NIH, AstraZeneca, Bristol-Myers Squibb, Michael J. Fox Foundation, Dystonia Medical Research Foundation, CHDI Foundation, and Thome Memorial Foundation. Over the past three years he has served on the Scientific Advisory Boards for Michael J. Fox Foundation, Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and Neurobiology Foundation for Schizophrenia and Bipolar Disorder. SEY, DJF, DPC, MSM, JG, PMGB, HPC, MB, ALB, MEJ, JFC, and CKJ declare no potential conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yohn, S.E., Foster, D.J., Covey, D.P. et al. Activation of the mGlu1 metabotropic glutamate receptor has antipsychotic-like effects and is required for efficacy of M4 muscarinic receptor allosteric modulators. Mol Psychiatry 25, 2786–2799 (2020). https://doi.org/10.1038/s41380-018-0206-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-018-0206-2
This article is cited by
-
Identifying drug targets for schizophrenia through gene prioritization
Translational Psychiatry (2026)
-
Overexpression of mGlu7B in Mice: Implications for Neurodevelopmental Disorders
Molecular Neurobiology (2025)
-
Evolutionary and functional analysis of metabotropic glutamate receptors in lampreys
Fish Physiology and Biochemistry (2024)
-
Current Findings and Potential Mechanisms of KarXT (Xanomeline–Trospium) in Schizophrenia Treatment
Clinical Drug Investigation (2024)
-
Enriched Environment Contributes to the Recovery from Neurotoxin-Induced Parkinson’s Disease Pathology
Molecular Neurobiology (2024)