Abstract
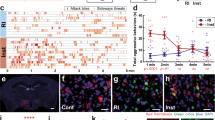
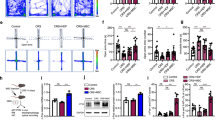
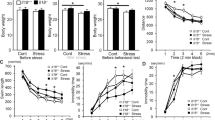
Heightened aggressive behavior is considered as one of the central symptoms of many neuropsychiatric disorders including autism, schizophrenia, and dementia. The consequences of aggression pose a heavy burden on patients and their families and clinicians. Unfortunately, we have limited treatment options for aggression and lack mechanistic insight into the causes of aggression needed to inform new efforts in drug discovery and development. Levels of proinflammatory cytokines in the periphery or cerebrospinal fluid were previously reported to correlate with aggressive traits in humans. However, it is still unknown whether cytokines affect brain circuits to modulate aggression. Here, we examined the functional role of interleukin 1β (IL-1β) in mediating individual differences in aggression using a resident-intruder mouse model. We found that nonaggressive mice exhibit higher levels of IL-1β in the dorsal raphe nucleus (DRN), the major source of forebrain serotonin (5-HT), compared to aggressive mice. We then examined the effect of pharmacological antagonism and viral-mediated gene knockdown of the receptors for IL-1 within the DRN and found that both treatments consistently increased aggressive behavior of male mice. Aggressive mice also exhibited higher c-Fos expression in 5-HT neurons in the DRN compared to nonaggressive mice. In line with these findings, deletion of IL-1 receptor in the DRN enhanced c-Fos expression in 5-HT neurons during aggressive encounters, suggesting that modulation of 5-HT neuronal activity by IL-1β signaling in the DRN controls expression of aggressive behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38.
Maynard Smith J, Price GR. The logic of animal conflict. Nature. 1973;246:15–8.
Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–64.
Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain Behav Immun. 2006;20:507–14.
Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–7.
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53.
Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–54.
Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci. 2014;111:16136–41.
Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20:1752–60.
Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep. 2017;7:12838.
Takahashi A, Flanigan ME, McEwen BS, Russo SJ. Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci. 2018;12:56.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15:371–87.
Cirulli F, De Acetis L, Alleva E. Behavioral effects of peripheral interleukin-1 administration in adult CD-1 mice: specific inhibition of the offensive components of intermale agonistic behavior. Brain Res. 1998;791:308–12.
Coccaro EF, Lee R, Coussons-Read M. Cerebrospinal fluid inflammatory cytokines and aggression in personality disordered subjects. Int J Neuropsychopharmacol. 2015;18:pyv001.
Pesce M, Speranza L, Franceschelli S, Ialenti V, Iezzi I, Patruno A, et al. Positive correlation between serum interleukin-1β and state anger in rugby athletes. Aggress Behav. 2013;39:141–8.
Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology. 2011;213:243–64.
Jacobs BL, Cohen A. Differential behavioral effects of lesions of the median or dorsal raphe nuclei in rats: open field and pain-elicited aggression. J Comp Physiol Psychol. 1976;90:102–8.
van der Vegt BJ, Lieuwes N, van de Wall EHEM, Kato K, Moya-Albiol L, Martínez-Sanchis S, et al. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–74.
Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193:295–304.
Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–80.
Faccidomo S, Quadros IMH, Takahashi A, Fish EW, Miczek KA. Infralimbic and dorsal raphé microinjection of the 5-HT(1B) receptor agonist CP-93,129: attenuation of aggressive behavior in CFW male mice. Psychopharmacology. 2012;222:117–28.
Takahashi A, Lee RX, Iwasato T, Itohara S, Arima H, Bettler B, et al. Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J Neurosci. 2015;35:6452–63.
Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–92.
Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 2017;16:44–55.
Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, et al. Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat Neurosci. 2020;23:638–50.
Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–59.
Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57:47–55.
Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–60.
Hen R. Mean genes. Neuron 1996;16:17–21.
Coccaro EF, Kavoussi RJ, Trestman RL, Gabriel SM, Cooper TB, Siever LJ. Serotonin function in human subjects: intercorrelations among central 5-HT indices and aggressiveness. Psychiatry Res. 1997;73:1–14.
Olivier B. Serotonin and aggression. Ann N Y Acad Sci. 2004;1036:382–92.
de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharm. 2005;526:125–39.
Takahashi A, Quadros IM, de Almeida RMM, Miczek KA. Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology. 2011;213:183–212.
Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology. 2015;40:88–112.
Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–9.
Brambilla D, Franciosi S, Opp MR, Imeri L. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur J Neurosci. 2007;26:1862–9.
Glaser R, Kiecolt-Glaser JK. Science and society: stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51.
Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–8.
Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–8.
Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12.
Loddick SA, Liu C, Takao T, Hashimoto K, De Souza EB. Interleukin-1 receptors: cloning studies and role in central nervous system disorders. Brain Res Rev. 1998;26:306–19.
Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: Biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–25.
Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–70.
Suzuki E. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17:557–62.
Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 1998;18:2239–46.
Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78:38–48.
Bluthé RM, Dantzer R, Kelley KW. Central mediation of the effects of interleukin-1 on social exploration and body weight in mice. Psychoneuroendocrinology. 1997;22:1–11.
Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, et al. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–20.
Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–5.
Cunningham ET, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–14.
Schöbitz B, de Kloet ER, Holsboer F. Gene expression and function of interleukin I, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432.
Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: role of serotonin-2 receptors. Neuroscience. 2003;120:227–33.
Hassanain M, Bhatt S, Zalcman S, Siegel A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005;1048:1–11.
Linthorst AC, Flachskamm C, Müller-Preuss P, Holsboer F, Reul JM. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci. 1995;15:2920–34.
Linthorst AC, Flachskamm C, Holsboer F, Reul JM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology. 1994;135:520–32.
Gemma C, Imeri L, de Simoni MG, Mancia M. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol. 1997;272:R601–6.
Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210.
Desson SE, Ferguson AV. Interleukin 1β modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol. 2003;550:113–22.
Liu X, Quan N. Microglia and CNS Interleukin-1: beyond immunological concepts. Front Neurol. 2018;9:8.
Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40.
Srinivasan D, Yen JH, Joseph DJ, Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. 2004;24:6482–8.
Huang Y, Smith DE, Ibáñez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–59.
Acknowledgements
This research was supported by US National Institutes of Health Grants R01 MH090264-06 and R01 MH104559-02 to SJR. The study was also supported by JSPS KAKENHI Grant Number JP17H04766, JP15K12773, and JP19H05202 to AT. We would like to thank Dr Tsuyoshi Koide and Dr Yosuke Takei for sharing experimental equipment (realtime PCR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Takahashi, A., Aleyasin, H., Stavarache, M.A. et al. Neuromodulatory effect of interleukin 1β in the dorsal raphe nucleus on individual differences in aggression. Mol Psychiatry 27, 2563–2579 (2022). https://doi.org/10.1038/s41380-021-01110-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-021-01110-4
This article is cited by
-
A crucial role for the cortical amygdala in shaping social encounters
Nature (2025)
-
Lateral habenula glutamatergic neurons projecting to the dorsal raphe nucleus promote aggressive arousal in mice
Nature Communications (2022)
-
The neuroimmunology of social-stress-induced sensitization
Nature Immunology (2022)