Abstract
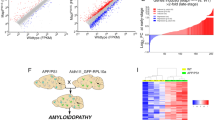
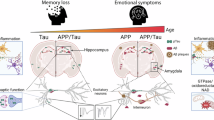
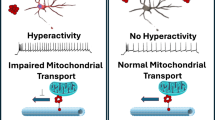
Protein aggregation in brainstem nuclei is thought to occur in the early stages of Alzheimer’s disease (AD), but its specific role in driving prodromal symptoms and disease progression is largely unknown. The dorsal raphe nucleus (DRN) contains a large population of serotonin (5-hydroxytryptamine; 5-HT) neurons that regulate mood, reward-related behavior, and sleep, which are all disrupted in AD. We report here that tau pathology is present in the DRN of individuals 25–80 years old without a known history of dementia, and its prevalence was comparable to the locus coeruleus (LC). By comparison, fewer cases were positive for other pathological proteins including α-synuclein, β-amyloid, and TDP-43. To evaluate how early tau pathology impacts behavior, we overexpressed human P301L-tau in the DRN of mice and observed depressive-like behaviors and hyperactivity without deficits in spatial memory. Tau pathology was predominantly found in neurons relative to glia and colocalized with a significant proportion of Tph2-expressing neurons in the DRN. 5-HT neurons were also hyperexcitable in P301L-tauDRN mice, and there was an increase in the amplitude of excitatory post-synaptic currents (EPSCs). Moreover, astrocytic density was elevated in the DRN and accompanied by an increase in IL-1α and Frk expression, which suggests increased inflammatory signaling. Additionally, tau pathology was detected in axonal processes in the thalamus, hypothalamus, amygdala, and caudate putamen. A significant proportion of this tau pathology colocalized with the serotonin reuptake transporter (SERT), suggesting that tau may spread in an anterograde manner to regions outside the DRN. Together these results indicate that tau pathology accumulates in the DRN in a subset of individuals over 50 years and may lead to behavioral dysregulation, 5-HT neuronal dysfunction, and activation of local astrocytes which may be prodromal indicators of AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR. Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol. 2009;35:532–54.
Michelsen KA, Prickaerts J, Steinbusch HWM. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–64.
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–83.
Wise EA, Rosenberg PB, Lyketsos CG, Leoutsakos J-M. Time course of neuropsychiatric symptoms and cognitive diagnosis in National Alzheimer’s Coordinating Centers volunteers. Alzheimers Dement. 2019;11:333–9.
Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJH, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–8.
Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Loñ L, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70:584–92.
Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res. 2008;5:342–5.
Theofilas P, Dunlop S, Heinsen H, Grinberg LT. Turning on the light within: subcortical nuclei of the isodentritic core and their role in Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2015;46:17–34.
Oh J, Eser RA, Ehrenberg AJ, Morales D, Petersen C, Kudlacek J, et al. Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimers Dement. 2019. https://doi.org/10.1016/j.jalz.2019.06.3916.
Marcinkiewcz CA. Serotonergic systems in the pathophysiology of ethanol dependence: relevance to clinical alcoholism. ACS Chem Neurosci. 2015;2015:1026–39. https://doi.org/10.1021/cn5003573
Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600.
Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology. 2015. 2015. https://doi.org/10.1038/npp.2015.293.
Paul ED, Lowry CA. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol. 2013;27:1090–106.
Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell. 2018;175:472–487.e20.
Grinberg LT, Rüb U, Ferretti REL, Nitrini R, Farfel JM, Polichiso L, et al. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406–16.
Tejani-Butt SM, Yang J, Pawlyk AC. Altered serotonin transporter sites in Alzheimer’s disease raphe and hippocampus. Neuroreport. 1995;6:1207–10.
Kepe V, Barrio JR, Huang S-C, Ercoli L, Siddarth P, Shoghi-Jadid K, et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2006;103:702–7.
Chen CPLH, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol Appl Neurobiol. 2000;26:347–55.
Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–8.
Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, et al. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24:233–42.
Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O’Brien JT. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry. 2004;161:1096–102.
Benarroch EE, Schmeichel AM, Sandroni P, Parisi JE, Low PA. Rostral raphe involvement in Lewy body dementia and multiple system atrophy. Acta Neuropathol. 2007;114:213–20.
Grinberg LT, Rueb U, Alho ATdiL, Heinsen H. Brainstem pathology and non-motor symptoms in PD. J Neurol Sci. 2010;289:81–88.
Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. 2001;101:256–70.
Halliday GM, Mccann HL, Pamphlett R, Brooks WS, Creasey H, Mccusker E, et al. Brain stem serotonin-synthesizing neurons in Alzheimer’s disease: a clinicopathological correlation. Acta Neuropathol. 1992;84:638–50.
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9.
Cook C, Kang SS, Carlomagno Y, Lin W-L, Yue M, Kurti A, et al. Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model. Hum Mol Genet. 2015;24:6198–212.
Song L, Lu SX, Ouyang X, Melchor J, Lee J, Terracina G, et al. Analysis of tau post-translational modifications in rTg4510 mice, a model of tau pathology. Mol Neurodegener. 2015;10:14.
Iqbal K, Liu F, Gong C-X, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–64.
Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685.
Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34:989–98.
Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-250) estimated using the 2010 census. Neurology. 2013;80:1778–83.
Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 2015;1:103–10.
Torres Irizarry VC, Feng B, Yang X, Nirali P, Schaul S, Ibrahimi L, et al. Estrogen signaling in the dorsal raphe regulates binge-like drinking in mice. Transl Psychiatry. 2024;27:122.
Laurent C, Buée L, Blum D. Tau and neuroinflammation: what impact for Alzheimer’s Disease and Tauopathies? Biomed J. 2018;41:21–33.
Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–15.
Bretheau F, Castellanos-Molina A, Bélanger D, Kusik M, Mailhot B, Boisvert A, et al. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat Commun. 2022;13:5786.
Liu L, Xu Y, Liu Z, Chen J, Zhang Y, Zhu J, et al. IL12 polymorphisms, HBV infection and risk of hepatocellular carcinoma in a high-risk Chinese population. Int J Cancer. 2011;128:1692–6.
Zhu X-C, Tan L, Jiang T, Tan M-S, Zhang W, Yu J-T. Association of IL-12A and IL-12B polymorphisms with Alzheimer’s disease susceptibility in a Han Chinese population. J Neuroimmunol. 2014;274:180–4.
Khan KM, Balasubramanian N, Gaudencio G, Wang R, Selvakumar GP, Kolling L, et al. Human tau-overexpressing mice recapitulate brainstem involvement and neuropsychiatric features of early Alzheimer’s disease. Acta Neuropathol Commun. 2023;11:57.
Liu X, Zeng K, Li M, Wang Q, Liu R, Zhang B, et al. Expression of P301L-hTau in mouse MEC induces hippocampus-dependent memory deficit. Sci Rep. 2017;7:3914.
Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, Lee VM-Y. Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol. 2015;130:349–62.
Stratmann K, Heinsen H, Korf H-W, Del Turco D, Ghebremedhin E, Seidel K, et al. Precortical phase of Alzheimer’s disease (AD)-related tau cytoskeletal pathology. Brain Pathol. 2016;26:371–86.
Ehrenberg AJ, Kelberman MA, Liu KY, Dahl MJ, Weinshenker D, Falgàs N, et al. Priorities for research on neuromodulatory subcortical systems in Alzheimer’s disease: position paper from the NSS PIA of ISTAART. Alzheimer’s Dement. 2023. https://doi.org/10.1002/alz.12937.
Wood H. Menopause influences tau pathology. Nat Rev Neurol. 2022;18:317.
Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–65.
Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, Diberto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016. 2016. https://doi.org/10.1038/nature19318.
Hale MW, Shekhar A, Lowry CA. Development by environment interactions controlling tryptophan hydroxylase expression. J Chem Neuroanat. 2011;41:219–26.
Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–83.
Perroud N, Neidhart E, Petit B, Vessaz M, Laforge T, Relecom C, et al. Simultaneous analysis of serotonin transporter, tryptophan hydroxylase 1 and 2 gene expression in the ventral prefrontal cortex of suicide victims. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:909–18.
Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28.
Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13465:507–513.
Kulikova EA, Kulikov AV. Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: focus on animal models. Expert Opin Ther Targets. 2019;23:655–67.
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711.
Busche MA, Konnerth A. Neuronal hyperactivity-A key defect in Alzheimer’s disease? Bioessays. 2015;37:624–32.
Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–77.
Ding Z-B, Song L-J, Wang Q, Kumar G, Yan Y-Q, Ma C-G. Astrocytes: a double-edged sword in neurodegenerative diseases. Neural Regen Res. 2021;16:1702–10.
Bellot-Saez A, Kékesi O, Morley JW, Buskila Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci Biobehav Rev. 2017;77:87–97.
Mahmoud S, Gharagozloo M, Simard C, Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8:184.
Piacentini R, Li Puma DD, Mainardi M, Lazzarino G, Tavazzi B, Arancio O, et al. Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia. 2017;65:1302–16.
Goedert M, Crowther RA, Spillantini MG. Tau mutations cause frontotemporal dementias. Neuron. 1998;21:955–8.
Ferrer I, López-González I, Carmona M, Arregui L, Dalfó E, Torrejón-Escribano B, et al. Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol. 2014;73:81–97.
Howerton AR, Roland AV, Bale TL. Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J Neurosci. 2014;34:7113–23.
Perea JR, Ávila J, Bolós M. Dephosphorylated rather than hyperphosphorylated Tau triggers a pro-inflammatory profile in microglia through the p38 MAPK pathway. Exp Neurol. 2018;310:14–21.
Morales I, Jiménez JM, Mancilla M, Maccioni RB. Tau oligomers and fibrils induce activation of microglial cells. J Alzheimers Dis. 2013;37:849–56.
Aloisi F, Borsellino G, Caré A, Testa U, Gallo P, Russo G, et al. Cytokine regulation of astrocyte function: in-vitro studies using cells from the human brain. Int J Dev Neurosci. 1995;13:265–74.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7.
Kobayashi E, Nakano M, Kubota K, Himuro N, Mizoguchi S, Chikenji T, et al. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep. 2018;8:1712.
Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM-Y, et al. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26:644–54.
Maphis N, Xu G, Kokiko-Cochran ON, Jiang S, Cardona A, Ransohoff RM, et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–55.
Hopp SC, Lin Y, Oakley D, Roe AD, DeVos SL, Hanlon D, et al. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J Neuroinflammation. 2018;15:269.
Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–92.
Fleeman RM, Proctor EA. Astrocytic propagation of tau in the context of Alzheimer’s disease. Front Cell Neurosci. 2021;15:645233.
Vogels T, Murgoci A-N, Hromádka T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun. 2019;7:109.
Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 2019;142:2558–71.
Brendel M, Jaworska A, Probst F, Overhoff F, Korzhova V, Lindner S, et al. Small-animal PET imaging of tau pathology with 18F-THK5117 in 2 transgenic mouse models. J Nucl Med. 2016;57:792–8.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108.
Acknowledgements
We thank the Iowa Neurobank Core for providing human post-mortem tissues for this study, Mariah Leidinger of the Comparative Pathology Core at The University of Iowa for providing histology services for human and mouse tissues, and the personnel of the Department of Pathology’s Immunohistochemistry Lab at The University of Iowa for performing TDP-43 staining. We additionally thank Ramasamy Thangavel for performing the α-syn-AT8-TH triple stain in human LC tissue.
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA028931), the National Institute on Aging (R01 AG070841), and the Williams-Cannon Fellowship to CAM. SRP was supported by the National Institute of General Medical Sciences (T32 GM067795), KMK was supported by the National Institute of Diabetes and Digestive and Kidney Disease (T32 DK112751), and TDJ was supported by the National Heart, Lung, and Blood Institute (T32 HL007638). CAM, GA, and MMH also received generous support from the Roy J. and Lucille A Carver Trust for the “SMASH Dementia” Research Program of Excellence award which partially funded this project.
Author information
Authors and Affiliations
Contributions
CAM, GL, GA, and MMH conceived of the project and designed experiments. SRP, KLF, RW, NB, JR, KMK, TDJ, MLH, BJC, HRW, RB, MMH, and CAM performed experiments and contributed to data acquisition. CAM, SRP, KLF, RW, NB, JR, KMK, KD, GA, and MMH analyzed and interpreted the data. CAM and SRP wrote the original manuscript, and CAM, SRP, RW, NB, GA, and MMH edited the manuscript. CAM, GA, and MMH provided resources, and CAM was responsible for overall supervision. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have impacted or appeared to impact this work
Ethics approval
All procedures on mice in this study were approved by the Institutional Care and Use Committee at the University of Iowa.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pierson, S.R., Fiock, K.L., Wang, R. et al. Tau pathology in the dorsal raphe may be a prodromal indicator of Alzheimer’s disease. Mol Psychiatry 30, 532–546 (2025). https://doi.org/10.1038/s41380-024-02664-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02664-9
This article is cited by
-
Eagle eyes on the human brain and beyond: PET at ultra-high spatial resolution
European Journal of Nuclear Medicine and Molecular Imaging (2026)
-
Tau pathology in the brainstem monoaminergic neurons reflect resilience to Alzheimer’s disease pathology in the Nun study cases
Acta Neuropathologica Communications (2025)
-
Locus coeruleus tau validates and informs high-resolution MRI in aging and at earliest Alzheimer’s pathology stages
Acta Neuropathologica Communications (2025)
-
Chain-mediated effect of physical activity between Chinese language-based L2 motivational self-system and intended effort
Scientific Reports (2025)