Abstract
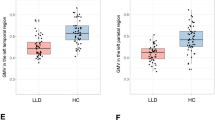
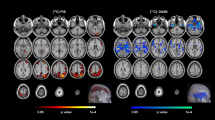
This study was conducted to clarify patterns of cortico-limbic volume abnormalities in late life depression (LLD) relative to non-depressed (ND) adults matched for amyloid β (Aβ) deposition and to evaluate the relationship of volume abnormalities with cognitive performance. Participants included 116 LLD and 226 ND. Classification accuracy of LLD status was estimated using area under the receiver operator characteristic curve. Twenty-one percent of LLD and ND participants were Aβ positive and the groups did not differ on white matter hyperintensity volume (WMH (logscale); β = 0.12, p = 0.28). Compared to ND, the LLD group exhibited significantly lower bilateral volume in the lateral orbitofrontal cortex, hippocampus, accumbens area, superior temporal lobe, temporal pole, and amygdala after multiple comparison correction (p < 0.009 for all). Cortico-limbic volumes significantly improved classification of LLD beyond demographic characteristics, Aβ status, and WMH (AUCVol = 0.71, AUCWMH, Aβ = 0.62, AUC difference, 0.09 [0.03 to 0.15]). LLD exhibited poorer performance on measures of global cognition, set shifting, and verbal learning and memory relative to ND. Cognitive function was positively associated with cortico-limbic volumes and these relationships did not differ by group. Secondary analyses with an ND sample additionally matched for Mild Cognitive Impairment (MCI) diagnosis showed a similar but attenuated pattern of volume abnormalities. Overall, our results support LLD as being associated with cortico-limbic volume abnormalities that are distinct from Aβ and white matter pathologies and that these volume abnormalities are important factors associated with cognitive dysfunction in LLD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
De-identified data from participants who agreed to the distribution of data are available from the corresponding author upon reasonable request. Contact the corresponding author for more information.
References
Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–106.
Anstey KJ, von Sanden C, Sargent-Cox K, Luszcz MA. Prevalence and risk factors for depression in a longitudinal, population-based study including individuals in the community and residential care. Am J Geriatr Psychiatry. 2007;15:497–505. https://doi.org/10.1097/JGP.0b013e31802e21d8.
Penninx BW, Beekman AT, Deeg DJ, van Tilburg W. [Effects of depression on physical health and mortality in the elderly. Longitudinal results of the LASA research]. Tijdschr Gerontol Geriatr. 2000;31:211–8.
Lyness JM, Kim J, Tang W, Tu X, Conwell Y, King DA, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–23. https://doi.org/10.1097/01.JGP.0000235763.50230.83.
Sneed JR, Kasen S, Cohen P. Early-life risk factors for late-onset depression. Int J Geriatr Psychiatry. 2007;22:663–7. https://doi.org/10.1002/gps.1727.
Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. https://doi.org/10.1192/bjp.bp.112.118307.
Aziz R, Steffens DC. What are the causes of late-life depression? Psychiatric Clinics of N Am. 2013;36:497–516. https://doi.org/10.1016/j.psc.2013.08.001.
Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry. 2005;13:88–98. https://doi.org/10.1097/00019442-200502000-00002.
Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31. https://doi.org/10.1038/nrneurol.2011.60.
Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. https://doi.org/10.1097/JGP.0b013e3181b0fa13.
Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, et al. Gray matter changes in late life depression–a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–72. https://doi.org/10.1038/sj.npp.1301655.
Egger K, Schocke M, Weiss E, Auffinger S, Esterhammer R, Goebel G, et al. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164:237–44. https://doi.org/10.1016/j.pscychresns.2007.12.018.
Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. 2007;37:1763–73. https://doi.org/10.1017/S0033291707000128.
Lavretsky H, Roybal DJ, Ballmaier M, Toga AW, Kumar A. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–7.
Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, et al. Mapping brain size and cortical gray matter changes in elderly depression. Biol Psychiatry. 2004;55:382–9. https://doi.org/10.1016/j.biopsych.2003.09.004.
Almeida OP, Burton EJ, Ferrier N, McKeith IG, O’Brien JT. Depression with late onset is associated with right frontal lobe atrophy. Psychol Med. 2003;33:675–81.
Avedisova AS, Samotaeva IS, Luzin RV, Semenovyh NS, Sergunova KA, Akzhigitov RG, et al. [Apathy in depression: a morphometric analysis]. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119:141–7. https://doi.org/10.17116/jnevro2019119051141.
Taylor WD, Steffens DC, Payne ME, MacFall JR, Marchuk DA, Svenson IK, et al. Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late-life depression. Arch Gen Psychiatry. 2005;62:537–44. https://doi.org/10.1001/archpsyc.62.5.537.
Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43.
Steffens DC, McQuoid DR, Payne ME, Potter GG. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. https://doi.org/10.1097/JGP.0b013e3181d6c245.
Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–37. https://doi.org/10.1176/appi.ajp.2007.07030506.
De Winter FL, Emsell L, Bouckaert F, Claes L, Jain S, Farrar G, et al. No association of lower hippocampal volume with Alzheimer’s disease pathology in late-life depression. Am J Psychiatry. 2017;174:237–45. https://doi.org/10.1176/appi.ajp.2016.16030319.
Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, et al. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24:798–808. https://doi.org/10.1002/gps.2185.
Mackin RS, Tosun D, Mueller SG, Lee JY, Insel P, Schuff N, et al. Patterns of reduced cortical thickness in late-life depression and relationship to psychotherapeutic response. Am J Geriatr Psychiatry 2013;21;794–802.
Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ. Association of gray matter atrophy with age, beta-amyloid, and cognition in aging. Cerebral Cortex. 2014;24:1609–18. https://doi.org/10.1093/cercor/bht017.
Kawas CH, Greenia DE, Bullain SS, Clark CM, Pontecorvo MJ, Joshi AD, et al. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimers Dement. 2013;9:199–203. https://doi.org/10.1016/j.jalz.2012.06.005.
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. https://doi.org/10.1016/S1474-4422(13)70044-9.
Insel PS, Donohue MC, Berron D, Hansson O, Mattsson-Carlgren N. Time between milestone events in the Alzheimer’s disease amyloid cascade. Neuroimage. 2021;227:117676 https://doi.org/10.1016/j.neuroimage.2020.117676.
Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Någren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–60. https://doi.org/10.1212/WNL.0b013e3181b23564.
Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and β-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317–24. https://doi.org/10.1002/ana.21955.
Koivunen J, Scheinin N, Virta JR, Aalto S, Vahlberg T, Någren K, et al. Amyloid PET imaging in patients with mild cognitive impairment. Neurology. 2011;76:1085 https://doi.org/10.1212/WNL.0b013e318212015e.
Insel PS, Mattsson N, Donohue MC, Mackin RS, Aisen PS, Jack CR Jr, et al. The transitional association between β-amyloid pathology and regional brain atrophy. Alzheimers Dement. 2015;11:1171–9. https://doi.org/10.1016/j.jalz.2014.11.002.
Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. https://doi.org/10.1001/jamaneurol.2015.3098.
Alexopoulos GS. Role of executive function in late-life depression. J Clin Psychiatry. 2003;64:18–23.
Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, et al. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–50. https://doi.org/10.1002/gps.2030.
Gudmundsson P, Olesen PJ, Simoni M, Pantoni L, Ostling S, Kern S, et al. White matter lesions and temporal lobe atrophy related to incidence of both dementia and major depression in 70-year-olds followed over 10 years. Eur J Neurol. 2015;22:781–8.
Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. https://doi.org/10.1001/archpsyc.61.6.587.
Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65.
Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. Executive dysfunction and disability in elderly patients with major depression. Am J Geriatr Psychiatry. 2001;9:269–74.
Steffens DC, Hays JC, Krishnan KR. Disability in geriatric depression. Am J Geriatr Psychiatry. 1999;7:34–40.
King DA, Cox C, Lyness JM, Conwell Y, Caine ED. Quantitative and qualitative differences in the verbal learning performance of elderly depressives and healthy controls. J Int Neuropsychol Soc. 1998;4:115–26.
Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, et al. Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am J Geriatr Psychiatry. 2012;20:682–90. https://doi.org/10.1097/JGP.0b013e318246b6cb.
Kassel MT, Rhodes E, Insel PS, Woodworth K, Garrison-Diehn C, Satre DD, et al. Cognitive outcomes are differentially associated with depression severity trajectories during psychotherapy treatment for late life major depressive disorder. Int J Geriatr Psychiatry. 2002;37. https://doi.org/10.1002/gps.5779.
Siddarth P, Funes CM, Laird KT, Ercoli L, Lavretsky H. Predictors of Cognitive Improvement Following Treatment for Late-Life Depression. J Geriatr Psychiatry Neurol. 2021;34:162–8. https://doi.org/10.1177/0891988720915515.
Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. https://doi.org/10.1192/bjp.186.3.197.
Lim HK, Jung WS, Ahn KJ, Won WY, Hahn C, Lee SY, et al. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology. 2012;37:838–49. https://doi.org/10.1038/npp.2011.264.
Rhodes E, Insel PS, Butters MA, Morin R, Bickford D, Tosun D, et al. The impact of amyloid burden and APOE on rates of cognitive impairment in late life depression. J Alzheimers Dis. 2021;80:991–1002. https://doi.org/10.3233/JAD-201089.
Svenningsson AL, Stomrud E, Insel PS, Mattsson N, Palmqvist S, Hansson O. β-amyloid pathology and hippocampal atrophy are independently associated with memory function in cognitively healthy elderly. Sci Rep. 2019;9:11180 https://doi.org/10.1038/s41598-019-47638-y.
Oh H, Correia S, Salloway SP. Differential associations between PET and CSF Alzheimer’s disease biomarkers and cognition among cognitively normal older adults. Alzheimers Dement. 2020;16:e041577 https://doi.org/10.1002/alz.041577.
Insel PS, Mattsson N, Mackin RS, Schöll M, Nosheny RL, Tosun D, et al. Accelerating rates of cognitive decline and imaging markers associated with β-amyloid pathology. Neurology. 2016;86:1887–96. https://doi.org/10.1212/wnl.0000000000002683.
Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–32. https://doi.org/10.1176/appi.ajp.2007.07010175.
Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. https://doi.org/10.1093/pan/mpl013.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56.
Frances A. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging. 2009;21:239–51. https://doi.org/10.1007/978-3-642-02498-6_20.
Jagust WJ, Landau SM, Koeppe RA, Reiman EM, Chen K, Mathis CA, et al. The Alzheimer’s disease neuroimaging initiative 2 PET core: 2015. Alzheimers Dement. 2015;11:757–71. https://doi.org/10.1016/j.jalz.2015.05.001.
Mackin RS, Insel PS, Landau S, Bickford D, Morin R, Rhodes E, et al. Late-life depression is associated with reduced cortical amyloid burden: findings from the alzheimer’s disease neuroimaging initiative depression project. Biol Psychiatry. 2021;89:757–65. https://doi.org/10.1016/j.biopsych.2020.06.017.
Mackin RS, Insel PS, Landau S, Bickford D, Morin R, Rhodes E, et al. Late-life depression is associated with reduced cortical amyloid burden: findings from the Alzheimer’s disease neuroimaging initiative depression project. Biol Psychiatry. 2020. https://doi.org/10.1016/j.biopsych.2020.06.017.
Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74:826–36. https://doi.org/10.1002/ana.23908.
Saykin AJ, Shen L, Yao X, Kim S, Nho K, Risacher SL, et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimers Dement. 2015;11:792–814. https://doi.org/10.1016/j.jalz.2015.05.009.
Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24:709–11.
Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–8. https://doi.org/10.1002/ana.410070516.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodol). 1996;58:257–88.
Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. https://doi.org/10.1007/s00429-008-0189-x.
Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–5.
Krishnan KR, McDonald WM, Doraiswamy PM, Tupler LA, Husain M, Boyko OB, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–46. https://doi.org/10.1007/bf02191522.
Tadayonnejad R, Yang S, Kumar A, Ajilore O. Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS ONE. 2014;9:e96033 https://doi.org/10.1371/journal.pone.0096033.
Welstead M, Luciano M, Muniz-Terrera G, Saunders S, Mullin DS, Russ TC. Predictors of mild cognitive impairment stability, progression, or reversion in the Lothian birth cohort 1936. J Alzheimers Dis. 2021;80:225–32. https://doi.org/10.3233/JAD-201282.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Depression project (ADNI D) (National Institute of Mental Health Grant R01098062 and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). We acknowledge Ray and Dagmar Dolby Family Fund for research support and Avid Radiopharmaceuticals for providing Florbetapir for this study. Additional support was provided by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs (MKL, MTK, ER, RM). Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative-Depression project (ADNI-D) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) databases (www.loni .usc.edu). As such, the investigators within the ADNI D and ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI D investigators and ADNI investigators include http://adni.loni.usc.edu/study-design/ongoing-investigations/ ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of California, Los Angeles.
Author information
Authors and Affiliations
Contributions
SM, PI, DB, RM, DT, SL, MB, PA, RR, AS, AT, CJ, MW, CN made substantial contributions to the design and conceptualization of the study. SM, ER, EB, DB, MB, SL, PA, RR, DT, RK, CJ, AS, RM, CN, PI acquired the data. PI, SM, CN analyzed and interpreted the data. SM drafted the manuscript. ER, MK, MKL, EB, DB, RM, DT, SL, MB, PA, RR, AS, AT, RK, CJ, MW, CN and PI critically reviewed and revised the manuscript and added important intellectual content.
Corresponding author
Ethics declarations
Competing interests
During the past 2 years, RSM has received research support from The National Institute of Mental Health, the National Institute of Aging, and Johnson and Johnson. During the past 2 years, CN has been an advisor or consultant to Biohaven, Clexio Biosciences Ltd., Johnson and Johnson, Novartis, and Otsuka, has received royalties from UpToDate, and research support from National Institute of Mental Health. RM, ER, DT, AT, RR, CJ, RK, MK, MKL, MAB, EB, and DB reported no biomedical financial interests or potential competing interests. SL serves on DSMBs for KeifeRX and the NIH IPAT study. She has received speaking honoraria from Eisai and IMPACT-AD and serves as a consultant for Vaccinex and the eSMARTER trial. She is on the JAMA Neurology editorial board and receives research funding and other support from NIH and the Alzheimer’s Association. AJS receives support from multiple NIH grants and has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor); Bayer Oncology (Scientific Advisory Board); Eisai (Scientific Advisory Board); Siemens Medical Solutions USA, Inc. (Dementia Advisory Board); NIH NHLBI (MESA Observational Study Monitoring Board); Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). MWW has served on the Scientific Advisory Boards for Pfizer, BOLT International, Neurotrope Bioscience, Alzheon, Inc., Alzheimer’s Therapeutic Research Institute (ATRI), Eli Lilly, U. of Penn’s Neuroscience of Behavior Initiative, National Brain Research Centre (NBRC), India, Dolby Family Ventures, LP, and ADNI. PA has research grants from NIH, Lilly and Eisai, and consults with Merck, Roche, Genentech, Abbvie, Biogen, ImmunoBrain Checkpoint and Arrowhead. PSI consults with Roche and Merck.
Ethics approval and consent to participate
All participants provided written informed consent and all study procedures were approved by an Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mackin, R.S., Rhodes, E., Kassel, M. et al. Cortico-limbic volume abnormalities in late life depression are distinct from β amyloid and white matter pathologies. Mol Psychiatry 30, 1267–1276 (2025). https://doi.org/10.1038/s41380-024-02677-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02677-4
This article is cited by
-
Cerebral amyloid-β, glucose metabolism and hippocampal volume in major depression with and without suspected non-Alzheimer pathophysiology (SNAP)
Alzheimer's Research & Therapy (2025)