Abstract
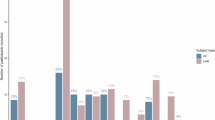
A novel radiotracer, [11C]SL25.1188, targets monoamine oxidase-B (MAO-B) enzyme, found primarily in astrocytes, which metabolizes monoamines (including dopamine), particularly in subcortical regions. Altered astrocyte function in schizophrenia is supported by convergent evidence from post-mortem, genetic, transcriptomic, peripheral and preclinical findings. We aimed to test whether levels of MAO-B, an index of astrocyte function are low in the living brains of early psychosis and their high-risk states. Thirty-eight participants including antipsychotic-free/minimally exposed clinical participants with first-episode psychosis (FEP), clinical high-risk (CHR) individuals and healthy volunteers (HVs) underwent a 90-min positron emission tomography (PET) scan with [11C]SL25.1188, to measure MAO-B VT, an index of MAO-B concentration. Participants were excluded if tested positive on urine drug screen (except for cannabis). This study of 14 FEP (mean[SD] age, 25.7[5.7] years; 6 F), 7 CHR (mean[SD] age, 20.9[3.7] years; 4 F) and 17 HV (mean[SD] age, 31.2[13.9] years; 9 F) demonstrated significant group differences in regional MAO-B VT (F(2,37.42) = 4.56, p = 0.02, Cohen’s f = 0.49), controlling for tobacco (F (1,37.42) = 5.37, p = 0.03) and cannabis use (F(1,37.42) = 5.11, p = 0.03) with significantly lower MAO-B VT in CHR compared to HV (Cohen’s d = 0.99). We report a significant cannabis effect on MAO-B VT (F(1,39.19) = 12.57, p = 0.001, Cohen’s f = 0.57), with a significant group-by-cannabis interaction (F(2,37.30) = 3.82, p = 0.03, Cohen’s f = 0.45), indicating lower MAO-B VT in cannabis-using clinical groups. Lower MAO-B VT levels were more robust in striatal than cortical regions, in both clinical groups (F(12,46.84) = 2.08, p = 0.04, Cohen’s f = 0.73) and in cannabis users (F(6,46.84) = 6.42, p < 0.001, Cohen’s f = 0.91). Lower MAO-B concentration supports astrocyte dysfunction in cannabis-using CHR and FEP clinical populations. Lower MAO-B is consistent with replicated striatal dopamine elevation in psychosis, as well as astrocyte dysfunction in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Anonymized individual participant data are available in the Supplementary Appendix.
Code availability
Analysis codes are available in the Supplementary Appendix.
References
Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40.
Bosworth AP, Allen NJ. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol. 2017;47:38–43.
Mei YY, Wu DC, Zhou N. Astrocytic regulation of glutamate transmission in Schizophrenia. Front Psychiatry. 2018;9:544.
Petrelli F, Dallérac G, Pucci L, Calì C, Zehnder T, Sultan S, et al. Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol Psychiatry. 2020;25:732–49.
Chen Y, Qin C, Huang J, Tang X, Liu C, Huang K, et al. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif. 2020;53:e12781.
Notter T. Astrocytes in schizophrenia. Brain Neurosci Adv. 2021;5:23982128211009148.
de Oliveira Figueiredo EC, Calì C, Petrelli F, Bezzi P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia. 2022;70:1585–604.
Kim M, Choi W, Choi S, Oh H, Kim J, Lee J, et al. In vivo reactive astrocyte imaging in patients with schizophrenia using fluorine 18–Labeled THK5351. JAMA Netw Open. 2024;7:e2410684.
Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, Shih JC, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70:755–74.
Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–99.
Glover V, Sandler M, Owen F, Riley GJ. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977;265:80–1.
Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab. 2013;33:863–71.
Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–7.
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86.
Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39:1479–89.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20.
Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75.
Nam MH, Sa M, Ju YH, Park MG, Lee CJ. Revisiting the role of Astrocytic MAOB in Parkinson’s Disease. Int J Mol Sci. 2022;23:4453.
Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62:15–30.
Tong J, Rathitharan G, Meyer JH, Furukawa Y, Ang LC, Boileau I, et al. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain. 2017;140:2460–74.
Fowler CJ, Carlsson A, Winblad B. Monoamine oxidase-A and -B activities in the brain stem of schizophrenics and non-schizophrenic psychotics. J Neural Transm. 1981;52:23–32.
Owen F, Crow TJ, Frith CD, Johnson JA, Johnstone EC, Lofthouse R, et al. Selective decreases in MAO-B activity in post-mortem brains from schizophrenic patients with type II syndrome. Br J Psychiatry. 1987;151:514–9.
Mann JJ, Kaplan RD, Bird ED. Elevated postmortem monoamine oxidase B activity in the caudate nucleus in Huntington’s disease compared to schizophrenics and controls. J Neural Transm. 1986;65:277–83.
Glover ReveleyMA, Sandler V, Spokes M. EG. Brain monoamine oxidase activity in schizophrenics and controls. Arch Gen Psychiatry. 1981;38:663–5.
Schwartz MA, Wyatt RJ, Yang HY, Neff NH. Multiple forms of brain monoamine oxidase in schizophrenic and normal individuals. Arch Gen Psychiatry. 1974;31:557–60.
Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z J Psychiatry. 2014;48:722–34.
Schümberg K, Polyakova M, Steiner J, Schroeter ML. Serum S100B is related to illness duration and clinical symptoms in schizophrenia—a meta-regression analysis. Front Cell Neurosci. 2016;10:46.
Williams M, Pearce RKB, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:357–62.
Williams MR, Galvin K, O’Sullivan B, MacDonald CD, Ching EWK, Turkheimer F, et al. Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci. 2014;264:285–96.
Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in Schizophrenia. Schizophr Res. 2008;103:71–82.
Nisha Aji K, Meyer JH, Rusjan PM, Mizrahi R. Monoamine Oxidase B (MAO-B): a target for rational drug development in schizophrenia using PET imaging as an example. Adv Neurobiol. 2023;30:335–62.
Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–6.
Holt LM, Nestler EJ. Astrocytic transcriptional and epigenetic mechanisms of drug addiction. J Neural Transm. 2024;131:409–24.
Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schyler D, et al. Slow recovery of human brain MAO B after L-deprenyl (Selegeline) withdrawal. Synapse. 1994;18:86–93.
Saba W, Valette H, Peyronneau MA, Bramoullé Y, Coulon C, Curet O, et al. [(11)C]SL25.1188, a new reversible radioligand to study the monoamine oxidase type B with PET: preclinical characterisation in nonhuman primate. Synapse. 2010;64:61–9.
Gates KS, Silverman RB. 5-(Aminomethyl)-3-aryl-2-oxazolidinones. A novel class of mechanism-based inactivators of monoamine oxidase B. J Am Chem Soc. 1990;112:9364–72.
Bramoullé Y, Puech F, Saba W, Valette H, Bottlaender M, George P. et al. Radiosynthesis of (S)-5-methoxymethyl-3-[6-(4,4,4-trifluorobutoxy)benzo[d]isoxazol-3-yl] oxazolidin-2-[11C]one ([11C]SL25.1188), a novel radioligand for imaging monoamine oxidase-B with PET. J Label Compd Radiopharm. 2008;51:153–8.
Rusjan PM, Wilson AA, Miler L, Fan I, Mizrahi R, Houle S, et al. Kinetic modeling of the monoamine oxidase B radioligand [11C]SL25.1188 in human brain with high-resolution positron emission tomography. J Cereb Blood Flow Metab. 2014;34:883–9.
Curet O, Sontag N, Aubin N, Marc C, Jegham S, George PB, et al. Biochemical profile of SL25.1188, a new reversi- ble MAO-B inhibitor. In Proceedings of the 29th annual meeting of society neuroscience. 1999. p. 848–14.
Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of Schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–13.
McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33.
Plavén-Sigray P, Matheson GJ, Coughlin JM, Hafizi S, Laurikainen H, Ottoy J, et al. Meta-analysis of the Glial Marker TSPO in psychosis revisited: reconciling inconclusive findings of patient–control differences. Biol Psychiatry. 2021;89:e5–8.
Landucci E, Mazzantini C, Lana D, Giovannini MG, Pellegrini-Giampietro DE. Neuronal and astrocytic morphological alterations driven by prolonged exposure with Δ9-Tetrahydrocannabinol but not cannabidiol. Toxics. 2022;10:48.
Dosumu O, Owolabi O, Ugbaja R, Popoola A, Rotimi S, Taiwo O, et al. Administration of cannabis causes alterations in monoamine oxidase B and serotonin receptor 2c gene expressions in wistar rats. Acta Facultatis Medicae Naissensis. 2021;38:35–46.
Fisar Z. Inhibition of monoamine oxidase activity by cannabinoids. Naunyn-Schmiedeberg’s. Archiv Pharmacol. 2010;381:563–72.
McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the locus of dopaminergic dysfunction in Schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44:1301–11.
Maitra M, Mitsuhashi H, Rahimian R, Chawla A, Yang J, Fiori LM, et al. Cell type specific transcriptomic differences in depression show similar patterns between males and females but implicate distinct cell types and genes. Nat Commun. 2023;14:2912.
Dehestani M, Kozareva V, Blauwendraat C, Fraenkel E, Gasser T, Bansal V. Transcriptomic changes in oligodendrocytes and precursor cells associate with clinical outcomes of Parkinson’s disease. Mol Brain. 2024;17:56.
Malaiya S, Cortes-Gutierrez M, Herb BR, Coffey SR, Legg SRW, Cantle JP, et al. Single-nucleus RNA-Seq reveals dysregulation of striatal cell identity due to huntington’s disease mutations. J Neurosci. 2021;41:5534–52.
Gill T, Watling SE, Richardson JD, McCluskey T, Tong J, Meyer JH, et al. Imaging of astrocytes in posttraumatic stress disorder: a PET study with the monoamine oxidase B radioligand [11C]SL25.1188. Eur Neuropsychopharmacol. 2022;54:54–61.
Moriguchi S, Wilson AA, Miler L, Rusjan PM, Vasdev N, Kish SJ, et al. Monoamine Oxidase B total distribution volume in the prefrontal cortex of major depressive disorder: an [11C]SL25.1188 positron emission tomography study. JAMA Psychiatry. 2019;76:634–41. Jun 1
Koshimori Y, Cusimano MD, Vieira EL, Rusjan PM, Kish SJ, Vasdev N, et al. Astrogliosis marker 11C-SL25.1188 PET in traumatic brain injury with persistent symptoms. Brain. 2023;146:4469–75.
First MB, Williams JBW, KargRS, Spitzer RL. Structured Clinical Interview for DSM-5— Research Version(SCID-5 for DSM-5, Research Version; SCID-5-RV, Version 1.0.0). Arlington, VA, American Psychiatric Association, 2015.
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62.
Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–5.
First MB, Gibbon M. The structured clinical interview for DSM-IV Axis I Disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II Disorders (SCID-II). In: Comprehensive handbook of psychological assessment, Vol 2: Personality assessment. Hoboken, NJ, US: John Wiley & Sons, Inc.; 2004. p. 134–43.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Holzer L, Chinet L, Jaugey L, Plancherel B, Sofia C, Halfon O, et al. Detection of cognitive impairment with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in adolescents with psychotic symptomatology. Schizophr Res. 2007;95:48–53.
Rusjan P, Sabioni P, Di Ciano P, Mansouri E, Boileau I, Laveillé A, et al. Exploring occupancy of the histamine H3 receptor by pitolisant in humans using PET. Br J Pharmacol. 2020;177:3464–72.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ. et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300.
Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–6.
Bendikov I, Nadri C, Amar S, Panizzutti R, De MirandaJ, Wolosker H, et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res. 2007;90:41–51.
Watts JJ, Garani R, Da Silva T, Lalang N, Chavez S, Mizrahi R. Evidence that cannabis exposure, abuse, and dependence are related to glutamate metabolism and glial function in the anterior cingulate cortex: a 1H-Magnetic resonance spectroscopy study. Front Psychiatry. 2020;11:764.
Da Silva T, Hafizi S, Watts JJ, Weickert CS, Meyer JH, Houle S, et al. In vivo imaging of translocator protein in long-term cannabis users. JAMA Psychiatry. 2019;76:1305–13.
Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res. 2011;191:201–11.
Da Silva T, Wu A, Laksono I, Prce I, Maheandiran M, Kiang M, et al. Mitochondrial function in individuals at clinical high risk for psychosis. Sci Rep. 2018;8:6216.
Basavaraju R, Guo J, Small SA, Lieberman JA, Girgis RR, Provenzano FA. Hippocampal glutamate and positive symptom severity in clinical high risk for psychosis. JAMA Psychiatry. 2022;79:178–9.
Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017;9:25.
Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry. 2013;18:909–15.
Da Silva T, Hafizi S, Andreazza AC, Kiang M, Bagby RM, Navas E, et al. Glutathione, the major redox regulator, in the prefrontal cortex of individuals at clinical high risk for psychosis. Int J Neuropsychopharmacol. 2018;21:311–8.
Dwir D, Khadimallah I, Xin L, Rahman M, Du F, Öngür D, et al. Redox and immune signaling in schizophrenia: new therapeutic potential. Int J Neuropsychopharmacol. 2023;26:309–21.
Kopjar N, Fuchs N, Žunec S, Mikolić A, Micek V, Kozina G, et al. DNA damaging effects, oxidative stress responses and cholinesterase activity in blood and brain of wistar rats exposed to Δ9-Tetrahydrocannabinol. Molecules. 2019;24:1560.
Pietiläinen O, Trehan A, Meyer D, Mitchell J, Tegtmeyer M, Valakh V, et al. Astrocytic cell adhesion genes linked to schizophrenia correlate with synaptic programs in neurons. Cell Rep. 2023;42:111988.
Ling E, Nemesh J, Goldman M, Kamitaki N, Reed N, Handsaker RE, et al. A concerted neuron-astrocyte program declines in ageing and schizophrenia. Nature. 2024;627:604–11.
Levi L, Bar-Haim M, Winter-van Rossum I, Davidson M, Leucht S, Fleischhacker WW, et al. Cannabis use and symptomatic relapse in first episode schizophrenia: trigger or consequence? Data from the OPTIMISE study. Schizophr Bull. 2023;49:903–13.
Acknowledgements
This work was partially funded by Canadian Institutes of Health Research (CIHR) Grant (APP461645) and was done as part of the research undertaken thanks, in part, to funding from the Canada First Research Excellence Fund, awarded to the Healthy Brains for Healthy Lives initiative at McGill University to Dr. Mizrahi. The authors thank the staff of the FYPP clinic (Dr. Michael Kiang) for aiding in participant recruitment in Toronto and the PET Research Imaging Centre for the acquisition of data. We would also like to thank the participants and their families for their cooperation.
Author information
Authors and Affiliations
Contributions
Dr. Mizrahi and Dr. Nisha Aji had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Mizrahi. Acquisition, analysis, or interpretation of data: Lalang, Nisha Aji, Ramos-Jiménez, Rusjan, Mizrahi, Rahimian. Drafting of the manuscript: Nisha Aji, Mizrahi, Rahimian, Rusjan. Critical revision of the manuscript for important intellectual content: Nisha Aji, Mizrahi, Rusjan, Meyer, Boileau, Rahimian, Mechawar, Turecki, Chartrand. Statistical analysis: Nisha Aji, Mizrahi, Ramos-Jiménez. Obtained funding: Mizrahi. Administrative, technical, or material support: Rusjan, Chartrand. Supervision: Mizrahi, Rusjan.
Corresponding authors
Ethics declarations
Competing interests
NL is an employee at the Vertex Pharmaceuticals and the authors collectively declare that they have no financial relationships with commercial interests that could have appeared to influence the work reported in this paper. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nisha Aji, K., Lalang, N., Ramos-Jiménez, C. et al. Evidence of altered monoamine oxidase B, an astroglia marker, in early psychosis and high-risk state. Mol Psychiatry 30, 2049–2058 (2025). https://doi.org/10.1038/s41380-024-02816-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02816-x