Abstract
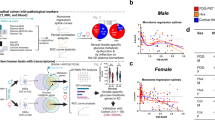
A major challenge in the development of more effective therapeutic strategies for Alzheimer’s disease (AD) is the identification of molecular mechanisms linked to specific pathophysiological features of the disease. Importantly AD has a two-fold higher incidence in women than men and a protracted prodromal phase characterized by amnestic mild-cognitive impairment (aMCI) suggesting that biological processes occurring early can initiate vulnerability to AD. Here, we used a sample of 125 subjects from two independent study cohorts to determine the levels in plasma (the most accessible specimen) of two essential mitochondrial markers acetyl-L-carnitine (LAC) and its derivative free-carnitine motivated by a mechanistic model in rodents in which targeting mitochondrial metabolism of LAC leads to the amelioration of cognitive function and boosts epigenetic mechanisms of gene expression. We report a sex-specific deficiency in free-carnitine levels in women with aMCI and early-AD compared to cognitively healthy controls; no change was observed in men. We also replicated the prior finding of decreased LAC levels in both women and men with AD, supporting the robustness of the study samples assayed in our new study. The magnitude of the sex-specific free-carnitine deficiency reflected the severity of cognitive dysfunction and held in two study cohorts. Furthermore, patients with the lower free-carnitine levels showed higher β-amyloid(Aβ) accumulation and t-Tau levels assayed in cerebrospinal fluid (CSF). Computational analyses showed that the mitochondrial markers assayed in plasma are at least as accurate as CSF measures to classify disease status. Together with the mechanistic platform in rodents, these translational findings lay the groundwork to create preventive individualized treatments targeting sex-specific changes in mitochondrial metabolism that may be subtle to early cognitive dysfunction of AD risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data will be made available upon request to the corresponding author Carla Nasca.
References
Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71.
Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. Alzheimer disease. Nat Rev Dis Prim. 2021;7:33.
Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48.
Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015;11:332–84.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Nasca C, Bigio B, Zelli D, de Angelis P, Lau T, Okamoto M, et al. Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron. 2017;96:402–413.e405.
Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA. 2013;110:4804–9.
Cuccurazzu B, Bortolotto V, Valente MM, Ubezio F, Koverech A, Canonico PL, et al. Upregulation of mGlu2 receptors via NF-kappaB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology. 2013;38:2220–30.
Bigio B, Mathe AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proc Natl Acad Sci USA. 2016;113:7906–11.
Morrison JH, Hof PR. Chapter 37 Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog Brain Res. 2002;136:467–86.
Fritz IB, McEwen BS. Effects of carnitine on fatty-acid oxidation by muscle. Science. 1959;129:334–5.
Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry. 2000;5:616–32.
Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, et al. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol Aging. 1990;11:499–506.
Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-l-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci USA. 2002;99:2356–61.
Liu J, Head E, Kuratsune H, Cotman CW, Ames BN. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann N Y Acad Sci. 2004;1033:117–31.
Gräff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111.
Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, Lambert G, et al. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc Natl Acad Sci USA. 2018;115:E11148–e11157.
Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer’s disease. Drugs. 2006;66:1515–34.
Nestler EJ, Peña CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic basis of mental illness. Neuroscientist. 2016;22:447–63.
Herzog CJ, Miot S, Mansuy IM, Giros B, Tzavara ET. Chronic valproate normalizes behavior in mice overexpressing calcineurin. Eur J Pharmacol. 2008;580:153–60.
Eriksson TM, Delagrange P, Spedding M, Popoli M, Mathé AA, Ögren SO, et al. Emotional memory impairments in a genetic rat model of depression: involvement of 5-HT/MEK/Arc signaling in restoration. Mol Psychiatry. 2012;17:173–84.
Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M, et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2021;26:5140–49.
De Felice FG, Gonçalves RA, Ferreira ST. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci. 2022;23:215–30.
De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106:1971–6.
Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG. Insulin resistance in Alzheimer’s disease. Front Neurosci. 2018;12:830.
Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–71.
Nasca C, Bigio B, Lee FS, Young SP, Kautz MM, Albright A, et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci USA. 2018;115:8627–32.
Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065.
Post RM. Myriad of implications of acetyl-l-carnitine deficits in depression. Proc Natl Acad Sci USA. 2018;115:8475–7.
Pfau ML, Russo SJ. Neuroinflammation regulates cognitive impairment in socially defeated mice. Trends Neurosci. 2016;39:353–5.
Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Mol Psychiatry. 2016;21:1648–52.
Cristofano A, Sapere N, La Marca G, Angiolillo A, Vitale M, Corbi G, et al. Serum levels of Acyl-carnitines along the continuum from normal to Alzheimer’s dementia. PLoS ONE. 2016;11:e0155694.
Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology. 1991;41:1726–32.
Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol Aging. 1995;16:1–4.
Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, et al. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology. 1996;47:705–11.
Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135:681–6.
Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol. 2015;6:103.
Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25:165–75.
Lourenco MV, Ribeiro FC, Sudo FK, Drummond C, Assunção N, Vanderborght B, et al. Cerebrospinal fluid irisin correlates with amyloid-β, BDNF, and cognition in Alzheimer’s disease. Alzheimers Dement. 2020;12:e12034.
Drummond C, Coutinho G, Fonseca RP, Assunção N, Teldeschi A, de Oliveira-Souza R, et al. Deficits in narrative discourse elicited by visual stimuli are already present in patients with mild cognitive impairment. Front Aging Neurosci. 2015;7:96.
Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6.
Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94.
Lourenco MV, Ribeiro FC, Santos LE, Beckman D, Melo HM, Sudo FK, et al. Cerebrospinal fluid neurotransmitters, cytokines, and chemokines in Alzheimer’s and lewy body diseases. J Alzheimers Dis. 2021;82:1067–74.
Nasca C, Barnhill O, DeAngelis P, Watson K, Lin J, Beasley J, et al. Multidimensional predictors of antidepressant responses: Integrating mitochondrial, genetic, metabolic and environmental factors with clinical outcomes. Neurobiol Stress. 2021;15:100407.
Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–80.
Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimer’s Dis. 2009;18:413–7.
Kapogiannis D, Mustapic M, Shardell MD, Berkowitz ST, Diehl TC, Spangler RD, et al. Association of extracellular vesicle biomarkers with Alzheimer disease in the Baltimore longitudinal study of aging. JAMA Neurol. 2019;76:1340–51.
Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, et al. Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res. 2002;70:357–60.
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophysica Acta (BBA) Mol Basis Dis. 2010;1802:2–10.
Smith MA, Perry G, Richey PL, Sayrec LM, Anderson VE, Beal MF, et al. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–1.
Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–8.
Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, et al. Neuropsychological Prediction of Conversion to Alzheimer Disease in Patients With Mild Cognitive Impairment. Arch Gen Psychiatry. 2006;63:916–24.
Acknowledgements
This work was supported by the National Institute on Aging (NIA) through the Early Adversity & Later Life Reversibility Pilot Grant to CN under Award Number R24AG06517, by a grant from the Robertson Therapeutic Development Foundation to CN, by New York University Langone fund to CN and by intramural grants from the D’Or Institute for Research and Education (IDOR) and Rede D’Or São Luiz Hospital Network to FTM. RASLF was supported by a predoctoral fellowship by FAPERJ and was awarded a CAEN 1A grant from the International Society for Neurochemistry and a PROLAB travel award from the American Society for Biochemistry and Molecular Biology. MVL was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (202.744/2019 and 010.002421/2019), Serrapilheira Institute (R-2012-37967, and Alzheimer’s Association (AARG-D-615741 and Blas Frangione Early Career Achievement Award). The UCI-ADRC is supported by NIH/NIA P50 AG16573 and P30AG066519. This paper is ad memoriam of Professor Bruce McEwen.
Author information
Authors and Affiliations
Contributions
Address correspondence to Carla Nasca (NYU School of Medicine, New York, email: carla.nasca@nyulangone.org) regarding the mechanistic platform, acetyl-L-carnitine (LAC) or free-carnitine and cohort samples; and to Mychael V. Lourenco (Institute of Medical Biochemistry Leopoldo de Meis, Rio de Janeiro, Brazil email: mychael@bioqmed.ufrj.br) regarding cohort samples. BB and CN conceived the mechanistic platform, statistical analyses, and computational approaches, and wrote the manuscript. RL, OB and MVL contributed to statistical analyses, interpretation of the data and manuscript writing. FKS, CD, NA, BV, FT-M, and PM contributed to patient recruitment, diagnosis, sample collection and cohort establishment. STF, BM and FGDF contributed reagents, materials or analysis tools and aided with interpretation of data. EH and DLS provided samples and clinical data from the UCI ADRC. JB, SY, AK, DJ performed LAC and carnitine assessment. BB and OB coordinated all experiments for LAC and carnitine assessment. CN supervised the research. All authors discussed and provided inputs to the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bigio, B., Lima-Filho, R.A.S., Barnhill, O. et al. Sex differences in mitochondrial free-carnitine levels in subjects at-risk and with Alzheimer’s disease in two independent study cohorts. Mol Psychiatry 30, 2573–2583 (2025). https://doi.org/10.1038/s41380-024-02862-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02862-5