Abstract
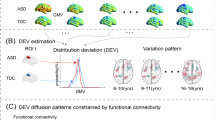
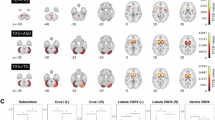
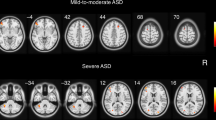
To understand the neural mechanism of autism spectrum disorder (ASD) and developmental delay/intellectual disability (DD/ID) that can be associated with ASD, it is important to investigate individuals at an early stage with brain, behavioural and also genetic measures, but such research is still lacking. Here, using the cross-sectional sMRI data of 1030 children under 8 years old, we employed developmental normative models to investigate the atypical development of gray matter volume (GMV) asymmetry in individuals with ASD without DD/ID, ASD with DD/ID and individuals with only DD/ID, and their associations with behavioral and clinical measures and transcription profiles. By extracting the individual deviations of patients from the typical controls with normative models, we found a commonly abnormal pattern of GMV asymmetry across all ASD children: more rightward laterality in the inferior parietal lobe and precentral gyrus, and higher individual variability in the temporal pole. Specifically, ASD with DD/ID children showed a severer and more extensive abnormal pattern in GMV asymmetry deviation values, which was linked with both ASD symptoms and verbal IQ. The abnormal pattern of ASD without DD/ID children showed higher and more extensive individual variability, which was linked with ASD symptoms only. DD/ID children showed no significant differences from healthy population in asymmetry. Lastly, the GMV laterality patterns of all patient groups were significantly associated with both shared and unique gene expression profiles. Our findings provide evidence for rightward GMV asymmetry of some cortical regions in young ASD children (1–7 years) in a large sample (1030 cases), show that these asymmetries are related to ASD symptoms, and identify genes that are significantly associated with these differences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Access to the identified participant research data must be approved by the research ethics board on a case-by-case basis, please contact the corresponding authors (feili@shsmu.edu.cn, mcao@fudan.edu.cn) for assistance in data access request.
Code availability
The code for spatial autocorrelation-preserving permutation test is available at (https://github.com/frantisekvasa/rotate_parcellation) (version 3, June 2022). All home developed codes will be given on request.
References
Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. 2013;21:591–643.
Lombardo MV, Lai M-C, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019;24:1435–50.
Li H-H, Feng J-Y, Wang B, Zhang Y, Wang C-X, Jia F-Y. Comparison of the children neuropsychological and behavior scale and the Griffiths mental development scales when assessing the development of children with autism. Psychol Res Behav Manag. 2019;12:973–81.
McDonald NM, Senturk D, Scheffler A, Brian JA, Carver LJ, Charman T, et al. Developmental trajectories of infants with multiplex family risk for autism: a baby siblings research consortium study. JAMA Neurol. 2020;77:73–81.
Srour M, Shevell M. Genetics and the investigation of developmental delay/intellectual disability. Arch Dis Child. 2014;99:386–9.
Shevell M, Ashwal S, Donley D, Flint J, Gingold M, Hirtz D, et al. Practice parameter: evaluation of the child with global developmental delay [RETIRED]: report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology. 2003;60:367–80.
Courchesne E, Pramparo T, Gazestani VH, Lombardo MV, Pierce K, Lewis NE. The ASD living Biology: from cell proliferation to clinical phenotype. Mol Psychiatry. 2019;24:88–107.
Lombardo MV, Pramparo T, Gazestani V, Warrier V, Bethlehem RA, Carter Barnes C, et al. Large-scale associations between the leukocyte transcriptome and BOLD responses to speech differ in autism early language outcome subtypes. Nat Neurosci. 2018;21:1680–8.
Courchesne E, Gazestani VH, Lewis NE. Prenatal origins of ASD: the when, what, and how of ASD development. Trends Neurosci. 2020;43:326–42.
Gazestani VH, Chiang AW, Courchesne E, Lewis NE. Autism genetics perturb prenatal neurodevelopment through a hierarchy of broadly-expressed and brain-specific genes. bioRxiv. 2020:2020.05. 23.112623.
Hyman SL, Levy SE, Myers SM, Kuo DZ, Apkon S, Davidson LF, et al. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447.
Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–37.
Cao M, Huang H, He Y. Developmental connectomics from infancy through early childhood. Trends Neurosci. 2017;40:494–506.
Brouwer RM, van Haren NE, Forti MD, van den Berg LH, Penninx BW, Falkai PG, et al. Dynamics of brain structure and its genetic architecture over the lifespan. Strukturelle und funktionelle Organisation des Gehirns; 2020.
Gottesman II, Hanson DR. Human development: biological and genetic processes. Annu Rev Psychol. 2005;56:263–86.
Parikshak NN, Gandal MJ, Geschwind DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet. 2015;16:441–58.
Güntürkün O, Ströckens F, Ocklenburg S. Brain lateralization: a comparative perspective. Physiol Rev. 2020;100:1019–63.
Wang J, Ma S, Yu P, He X. Evolution of human brain left–right asymmetry: old genes with new functions. Mol Biol Evol. 2023;40:msad181.
Roe JM, Vidal-Pineiro D, Amlien IK, Pan M, Sneve MH, de Schotten MT, et al. Tracing the development and lifespan change of population-level structural asymmetry in the cerebral cortex. Elife. 2023;12:e84685.
Carrion-Castillo A, Pepe A, Kong X-Z, Fisher SE, Mazoyer B, Tzourio-Mazoyer N, et al. Genetic effects on planum temporale asymmetry and their limited relevance to neurodevelopmental disorders, intelligence or educational attainment. Cortex. 2020;124:137–53.
Kong X-Z, Postema M, Schijven D, Castillo AC, Pepe A, Crivello F, et al. Large-scale phenomic and genomic analysis of brain asymmetrical skew. Cereb Cortex. 2021;31:4151–68.
Sha Z, Schijven D, Carrion-Castillo A, Joliot M, Mazoyer B, Fisher SE, et al. The genetic architecture of structural left–right asymmetry of the human brain. Nat Hum Behav. 2021;5:1226–39.
Ratnarajah N, Rifkin-Graboi A, Fortier MV, Chong YS, Kwek K, Saw S-M, et al. Structural connectivity asymmetry in the neonatal brain. Neuroimage. 2013;75:187–94.
Scheinost D, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, et al. Cerebral lateralization is protective in the very prematurely born. Cereb Cortex. 2015;25:1858–66.
Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 2012;22:13–25.
Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, et al. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex. 2011;21:1076–83.
Dubois J, Hertz-Pannier L, Cachia A, Mangin J-F, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19:414–23.
Li G, Nie J, Wang L, Shi F, Lyall AE, Lin W, et al. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 2014;24:1289–1300.
Koelkebeck K, Miyata J, Kubota M, Kohl W, Son S, Fukuyama H, et al. The contribution of cortical thickness and surface area to gray matter asymmetries in the healthy human brain. Hum Brain Mapp. 2014;35:6011–22.
Gracia-Tabuenca Z, Moreno MB, Barrios FA, Alcauter S. Hemispheric asymmetry and homotopy of resting state functional connectivity correlate with visuospatial abilities in school-age children. NeuroImage. 2018;174:441–8.
Song JW, Mitchell PD, Kolasinski J, Ellen Grant P, Galaburda AM, Takahashi E. Asymmetry of white matter pathways in developing human brains. Cereb Cortex. 2015;25:2883–93.
Shu N, Liu Y, Duan Y, Li K. Hemispheric asymmetry of human brain anatomical network revealed by diffusion tensor tractography. BioMed Res Int. 2015;2015:908917.
Zhong S, He Y, Shu H, Gong G. Developmental changes in topological asymmetry between hemispheric brain white matter networks from adolescence to young adulthood. Cereb Cortex. 2017;27:2560–70.
Karolis VR, Corbetta M, Thiebaut de Schotten M. The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat Commun. 2019;10:1–9.
Kleinhans NM, Müller R-A, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–25.
Joseph RM, Fricker Z, Fenoglio A, Lindgren KA, Knaus TA, Tager-Flusberg H. Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging Behav. 2014;8:60–72.
Floris DL, Lai MC, Auer T, Lombardo MV, Ecker C, Chakrabarti B, et al. Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Hum Brain Mapp. 2016;37:230–53.
Lindell AK, Hudry K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 2013;23:257–70.
Postema MC, Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun. 2019;10:1–12.
Dai Y, Liu Y, Zhang L, Ren T, Wang H, Yu J, et al. Shanghai autism early development: an integrative Chinese ASD cohort. Neurosci Bull. 2022;38:1603–7.
Zhao T, Liao X, Fonov VS, Wang Q, Men W, Wang Y, et al. Unbiased age-specific structural brain atlases for Chinese pediatric population. Neuroimage. 2019;189:55–70.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Fortin J-P, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Rutherford S, Kia SM, Wolfers T, Fraza C, Zabihi M, Dinga R, et al. The normative modeling framework for computational psychiatry. Nature protocols. 2022;17:1711–34.
Burt JB, Helmer M, Shinn M, Anticevic A, Murray JD. Generative modeling of brain maps with spatial autocorrelation. NeuroImage. 2020;220:117038.
Váša F, Seidlitz J, Romero-Garcia R, Whitaker KJ, Rosenthal G, Vértes PE, et al. Adolescent tuning of association cortex in human structural brain networks. Cereb Cortex. 2018;28:281–94.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–9.
Arnatkevic̆iūtė A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–67.
Abdi H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip Rev Comput Stat. 2010;2:97–106.
Romero-Garcia R, Seidlitz J, Whitaker KJ, Morgan SE, Fonagy P, Dolan RJ, et al. Schizotypy-related magnetization of cortex in healthy adolescence is colocated with expression of schizophrenia-related genes. Biol Psychiatry. 2020;88:248–59.
Xie Y, Xu Z, Xia M, Liu J, Shou X, Cui Z, et al. Alterations in connectome dynamics in autism spectrum disorder: a harmonized mega-and meta-analysis study using the autism brain imaging data exchange dataset. Biol Psychiatry. 2022;91:945–55.
Morgan SE, Seidlitz J, Whitaker KJ, Romero-Garcia R, Clifton NE, Scarpazza C, et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc Natl Acad Sci USA. 2019;116:9604–9.
Li J, Seidlitz J, Suckling J, Fan F, Ji G-J, Meng Y, et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun. 2021;12:1–14.
Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2014;88:212–27.
Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, et al. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. 2006;163:1440–3.
Rolls ET, Deco G, Huang C-C, Feng J. The human posterior parietal cortex: effective connectome, and its relation to function. Cereb Cortex. 2023;33:3142–70.
Yoshimura S, Sato W, Kochiyama T, Uono S, Sawada R, Kubota Y, et al. Gray matter volumes of early sensory regions are associated with individual differences in sensory processing. Hum Brain Mapp. 2017;38:6206–17.
Rolls ET, Deco G, Huang C-C, Feng J. Multiple cortical visual streams in humans. Cereb Cortex. 2023;33:3319–49.
Rolls ET, Deco G, Huang C-C, Feng J. The human language effective connectome. NeuroImage. 2022;258:119352.
Rolls ET. Brain computations and connectivity. Oxford University Press; 2023.
Fu L, Wang Y, Fang H, Xiao X, Xiao T, Li Y, et al. Longitudinal study of brain asymmetries in autism and developmental delays aged 2–5 years. Neuroscience. 2020;432:137–49.
Sha Z, Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, et al. Subtly altered topological asymmetry of brain structural covariance networks in autism spectrum disorder across 43 datasets from the ENIGMA consortium. Mol Psychiatry. 2022;27:2114–25.
Marquand AF, Rezek I, Buitelaar J, Beckmann CF. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80:552–61.
Wolfers T, Beckmann CF, Hoogman M, Buitelaar JK, Franke B, Marquand AF. Individual differences v. the average patient: mapping the heterogeneity in ADHD using normative models. Psychol Med. 2020;50:314–23.
Chien Y-L, Lin H-Y, Tung Y-H, Hwang T-J, Chen C-L, Wu C-S, et al. Neurodevelopmental model of schizophrenia revisited: similarity in individual deviation and idiosyncrasy from the normative model of whole-brain white matter tracts and shared brain-cognition covariation with ADHD and ASD. Mol Psychiatry. 2022;27:3262–71.
Pigdon L, Willmott C, Reilly S, Conti-Ramsden G, Gaser C, Connelly A, et al. Grey matter volume in developmental speech and language disorder. Brain Structure Funct. 2019;224:3387–98.
Aglinskas A, Hartshorne JK, Anzellotti S. Contrastive machine learning reveals the structure of neuroanatomical variation within autism. Science. 2022;376:1070–4.
Yamasaki S, Yamasue H, Abe O, Suga M, Yamada H, Inoue H, et al. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biol Psychiatry. 2010;68:1141–7.
Li C, Ning M, Fang P, Xu H. Sex differences in structural brain asymmetry of children with autism spectrum disorders. J Integr Neurosci. 2021;20:331–40.
Eisenberg IW, Wallace GL, Kenworthy L, Gotts SJ, Martin A. Insistence on sameness relates to increased covariance of gray matter structure in autism spectrum disorder. Mol Autism. 2015;6:1–12.
Rolls ET, Deco G, Huang C-C, Feng J. The connectivity of the human frontal pole cortex, and a theory of its involvement in exploit versus explore. Cereb Cortex. 2023;34:bhad416.
Indika N-LR, Deutz NE, Engelen MP, Peiris H, Wijetunge S, Perera R. Sulfur amino acid metabolism and related metabotypes of autism spectrum disorder: a review of biochemical evidence for a hypothesis. Biochimie. 2021;184:143–57.
van Sadelhoff JH, Perez Pardo P, Wu J, Garssen J, Van Bergenhenegouwen J, Hogenkamp A, et al. The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Front Endocrinol. 2019;10:247.
Brito NH, Noble KG. Socioeconomic status and structural brain development. Front Neurosci. 2014;8:276.
Rakesh D, Whittle S. Socioeconomic status and the developing brain–a systematic review of neuroimaging findings in youth. Neurosci Biobehav Rev. 2021;130:379–407.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81901826, 61932008, 62076068, 82271627, 82125032, 81930095, 81761128035, 82202243, and 82204048), the Science and Technology Commission of Shanghai Municipality (23Y21900500, 19410713500 and 2018SHZDZX01), the Shanghai Municipal Commission of Health and Family Planning (GWVI-11.1-34,GWV-10.1-XK07, 2020CXJQ01, 2018YJRC03), the Shanghai Clinical Key Subject Construction Project (shslczdzk02902), the Shanghai Municipal Science and Technology Major Project [2021ZD0200800,No.2018SHZDZX01], Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20211100), the Guangdong Key Project (2018B030335001), the Shanghai Municipal Commission of Health and Family Planning (20214Y0125), the National Key R&D Program of China (grant numbers 2018YFC1312900 and 2019YFA0709502), and the ZJ Lab, Shanghai Center for Brain Science and Brain-Inspired Technology and the 111 Project (grant number B18015).
Author information
Authors and Affiliations
Contributions
MC, FL and SG designed the study. YD, YL, YZ, LD, ZC contributed to the acquisition of research data. SG and MC conducted the data analysis. FL, MC, and SG provided the interpretation of results. MC and SG wrote the first draft of the manuscript. ETR and JF revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in compliance with the declaration of Helsinki, approved by the Ethics Committee of Xinhua Hospital affiliated with the Shanghai Jiao Tong University School of Medicine (XHEC-C-2019-076) and registered with ClinicalTrials.gov (NCT04358744). The legal guardian of all participants signed the written informed consent after detailed information notification.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Geng, S., Dai, Y., Rolls, E.T. et al. Rightward brain structural asymmetry in young children with autism. Mol Psychiatry 30, 2860–2870 (2025). https://doi.org/10.1038/s41380-025-02890-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02890-9
This article is cited by
-
Study on the Topological Properties of Language Brain Region Networks in Children With Autism Based on Diffusion Tensor Imaging (DTI)
Journal of Autism and Developmental Disorders (2026)
-
Neurite development varies across the hippocampus and covaries with the cellular composition of hippocampal tissue
Communications Biology (2025)