Abstract
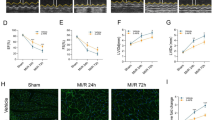
Clozapine is the only approved pharmacotherapy for treatment-resistant schizophrenia. However, widespread utilization of clozapine is constrained due to the potential for severe adverse effects, including myocarditis. Multiple mechanisms have been suggested to account for the cardiotoxic effects of clozapine, yet these investigations have not used cells derived from clozapine treated patients. In this study, cardiomyocytes that were derived from induced pluripotent stem cells generated from four patients with treatment-resistant schizophrenia with (n = 2) and without (n = 2) a history of clozapine-induced myocarditis were used to assess mitochondria- and NLRP3 inflammasome-mediated mechanisms of this severe adverse drug reaction. We found treatment of cardiomyocytes with a physiologically-relevant dose (2.8 µM) of clozapine for 24 h: (1) induced cardiac dysfunction, increased cytotoxicity, and apoptosis, (2) induced oxidative stress by elevating the level of reactive oxygen species and mitochondrial fragmentation, and (3) elevated levels of proinflammatory cytokines and activated the NLRP3 inflammasome. These effects were more pronounced in cardiomyocytes derived from individuals with a history of clozapine-induced myocarditis. Furthermore, pharmacological targeting of the mitochondria (elamipretide) and inflammasome (ustekinumab) attenuated these clozapine-induced cardiotoxic effects. Collectively, these results suggest a mitochondria- and NLRP3 inflammasome-mediated mechanism in the development of myocarditis associated with clozapine and support further evaluation of therapeutics that target mitochondria and NLRP3 signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. All relevant data have been presented in this article. There was no data excluded from the analysis.
References
Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60.
Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–26.
Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–7.
de Leon J, Ruan C-J, Schoretsanitis G, De las Cuevas C. A rational use of clozapine based on adverse drug reactions, pharmacokinetics, and clinical pharmacopsychology. Psychother Psychosom. 2020;89:200–14.
Cooper LT. Myocarditis. N Engl J Med. 2009;360:1526–38.
Remington G, Lee J, Agid O, Takeuchi H, Foussias G, Hahn M, et al. Clozapine’s critical role in treatment resistant schizophrenia: ensuring both safety and use. Expert Opin Drug Saf. 2016;15:1193–203.
Bellissima BL, Tingle MD, Cicović A, Alawami M, Kenedi C. A systematic review of clozapine-induced myocarditis. Int J Cardiol. 2018;259:122–9.
Vaziri N, Marques D, Greenway SC, Bousman CA. The cellular mechanism of antipsychotic-induced myocarditis: a systematic review. Schizophr Res. 2023;261:206–15.
Bousman C, Greenway SC, Tarailo-Graovac M, Long Q, Sellmer R, Crockford D, et al. editors. The pharmacogenomics of clozapine-induced myocarditis (PROCLAIM) consortium. Neuropsychopharmacology. 2018: Nature publishing group macmillan building, 4, England.
Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45:458–65.
Churko JM, Burridge PW, Wu JC. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods Mol Biol. 2013;1036:81–8.
Vaziri N, Marques D, Wang X, Machiraju P, Narang A, Vlahos K, et al. Generation of two human induced pluripotent stem cell lines from peripheral blood mononuclear cells of clozapine-tolerant and clozapine-induced myocarditis patients with treatment-resistant schizophrenia. Stem Cell Res. 2022;63:102877.
Machiraju P, Huang J, Iqbal F, Liu Y, Wang X, Bousman C, et al. Early inhibition of retinoic acid signaling rapidly generates cardiomyocytes expressing ventricular markers from human induced pluripotent stem cells. bioRxiv. 2019:856575.
Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–87.
Titier K, Canal M, Déridet E, Abouelfath A, Gromb S, Molimard M, et al. Determination of myocardium to plasma concentration ratios of five antipsychotic drugs: comparison with their ability to induce arrhythmia and sudden death in clinical practice. Toxicol Appl Pharmacol. 2004;199:52–60.
Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62.
Wu J, Hao S, Sun X-R, Zhang H, Li H, Zhao H, et al. Elamipretide (SS-31) ameliorates isoflurane-induced long-term impairments of mitochondrial morphogenesis and cognition in developing rats. Front Cell Neurosci. 2017;11:119.
Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, et al. Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci. 2020;117:15363–73.
Szeto HH. First‐in‐class cardiolipin‐protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171:2029–50.
Machiraju P, Wang X, Sabouny R, Huang J, Zhao T, Iqbal F, et al. SS-31 peptide reverses the mitochondrial fragmentation present in fibroblasts from patients with DCMA, a mitochondrial cardiomyopathy. Front Cardiovasc Med. 2019;6:167.
Rohani L, Machiraju P, Sabouny R, Meng G, Liu S, Zhao T, et al. Reversible mitochondrial fragmentation in iPSC-derived cardiomyocytes from children with DCMA, a mitochondrial cardiomyopathy. Can J Cardiol. 2020;36:554–63.
Chetwood J, Gupta S, Subramaniam K, De Cruz P, Moore G, An Y, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis–national extended follow-up and a review of the literature. Expert Opin Drug Saf. 2024;23:449–56.
Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006;18:1115–26.
Sala L, Van Meer BJ, Tertoolen LG, Bakkers J, Bellin M, Davis RP, et al. MUSCLEMOTION: a versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circ Res. 2018;122:e5–e16.
Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–13.
Zhang F, Han L, Wang J, Shu M, Liu K, Zhang Y, et al. Clozapine induced developmental and cardiac toxicity on Zebrafish Embryos by elevating oxidative stress. Cardiovasc Toxicol. 2021;21:399–409.
Abdel-Wahab BA, Metwally ME. Clozapine-induced cardiotoxicity in rats: involvement of tumour necrosis factor alpha, NF-κβ and caspase-3. Toxicol Rep. 2014;1:1213–23.
Abdel-Wahab BA, Metwally ME. Clozapine-induced cardiotoxicity: role of oxidative stress, tumour necrosis factor alpha and NF-κβ. Cardiovasc Toxicol. 2015;15:355–65.
Hayes RD, Downs J, Chang C-K, Jackson RG, Shetty H, Broadbent M, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41:644–55.
Yusufi B, Mukherjee S, Flanagan R, Paton C, Dunn G, Page E, et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol. 2007;22:238–43.
Nilsson BM, Edström O, Lindström L, Wernegren P, Bodén R. Tachycardia in patients treated with clozapine versus antipsychotic long-acting injections. Int Clin Psychopharmacol. 2017;32:219–24.
Kawano H, Okada R, Kawano Y, Sueyoshi N, Shirai T. Apoptosis in acute and chronic myocarditis. Jpn Heart J. 1994;35:745–50.
Scarabelli TM, Knight R, Stephanou A, Townsend P, Chen-Scarabelli C, Lawrence K, et al. Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol. 2006;31:181–264.
Liu P, Sole MJ. What is the relevance of apoptosis to the myocardium? Can J Cardiol. 1999;15:8B–10B.
Shi Y. Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci. 2004;13:1979–87.
Jarskog LF, Gilmore JH, Glantz LA, Gable KL, German TT, Tong RI, et al. Caspase-3 activation in rat frontal cortex following treatment with typical and atypical antipsychotics. Neuropsychopharmacology. 2007;32:95–102.
Arends M, Morris R, Wyllie A. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136:593.
Yuan J, Lipinski M, Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–13.
Scheffer DdL, Garcia AA, Lee L, Mochly-Rosen D, Ferreira JCB. Mitochondrial fusion, fission, and mitophagy in cardiac diseases: challenges and therapeutic opportunities. Antioxid Redox Signal. 2022;36:844–63.
Ben‐Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J Neurochem. 2002;83:1241–51.
Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610.
Kohen R, Nyska A. Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50.
Martín-Fernández B, Gredilla R. Mitochondria and oxidative stress in heart aging. Age. 2016;38:225–38.
Hafez AA, Jamali Z, Khezri S, Salimi A. Thymoquinone reduces mitochondrial damage and death of cardiomyocytes induced by clozapine. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:1675–84.
Mishra P, Samanta L. Oxidative stress and heart failure in altered thyroid states. Sci World J. 2012;2012:741861.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
Kingston E, Tingle M, Bellissima BL, Helsby N, Burns K. CYP-catalysed cycling of clozapine and clozapine-N-oxide promotes the generation of reactive oxygen species in vitro. Xenobiotica. 2024;54:26–37.
Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, et al. Mitochondrial‐targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–71.
Liu Y, Yang W, Sun X, Xie L, Yang Y, Sang M, et al. SS31 ameliorates sepsis-induced heart injury by inhibiting oxidative stress and inflammation. Inflammation. 2019;42:2170–80.
Jeong S-Y, Seol D-W. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22.
Li L, Gao P, Tang X, Liu Z, Cao M, Luo R, et al. CB1R-stabilized NLRP3 inflammasome drives antipsychotics cardiotoxicity. Signal Transduct Target Ther. 2022;7:1–15.
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7.
Rauf A, Shah M, Yellon DM, Davidson SM. Role of caspase 1 in ischemia/reperfusion injury of the myocardium. J Cardiovasc Pharmacol. 2019;74:194–200.
Wang J-F, Min J-Y, Hampton TG, Amende I, Yan X, Malek S, et al. Clozapine-induced myocarditis: role of catecholamines in a murine model. Eur J Pharmacol. 2008;592:123–7.
Hu X, Ma R, Lu J, Zhang K, Xu W, Jiang H, et al. IL-23 promotes myocardial I/R injury by increasing the inflammatory responses and oxidative stress reactions. Cell Physiol Biochem. 2016;38:2163–72.
Afanasyeva M, Wang Y, Kaya Z, Stafford EA, Dohmen KM, Sadighi Akha AA, et al. Interleukin-12 receptor/STAT4 signaling is required for the development of autoimmune myocarditis in mice by an interferon-γ–independent pathway. Circulation. 2001;104:3145–51.
Jimenez-Tellez N, Greenway SC. Cellular models for human cardiomyopathy: what is the best option? World J Cardiol. 2019;11:221.
Musunuru K, Sheikh F, Gupta RM, Houser SR, Maher KO, Milan DJ, et al. Induced pluripotent stem cells for cardiovascular disease modeling and precision medicine: a scientific statement from the american heart association. Circ Genom Precis Med. 2018;11:e000043.
Acknowledgements
This work was supported by William H. Davies Medical Research Scholarships, Calgary Chapter of the Schizophrenia Society of Alberta, Dr. S.K. Littman Graduate Award, Libin Cardiovascular Institute BRAIN CREATE Doctoral Scholarship, Maria Fotaki International Student Graduate Scholarship, and Alberta Children Hospital (ACHRI) graduate scholarship. The human iPSCs were obtained from Joseph C. Wu, MD, PhD, Stanford Cardiovascular Institute. We acknowledge the Hotchkiss Brain Institute Advanced Microscopy Platform and the Cumming School of Medicine for support and use of slide scanner microscope and Charbonneau Microscopy Facility for support and use of confocal microscope. C Pantelis was supported by a National Health and Medical Research Council (NHMRC) L3 Investigator Grant (1196508) and NHMRC Program Grant (ID: 1150083).
Author information
Authors and Affiliations
Contributions
NV, CAB: Writing - Original Draft; CAB, SCG, TES: Conceptualization; NV: Methodology and Formal Analysis; TES, WK, TJR, CP, NT, MJ, SCG: Review & Editing.
Corresponding author
Ethics declarations
Competing interests
CAB is founder of Sequence2Script Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41380_2025_2935_MOESM1_ESM.docx
Examination of mitochondria- and inflammasome-mediated mechanisms of clozapine-induced myocarditis using patient-derived iPSC cardiomyocytes
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaziri, N., Shutt, T.E., Karim, W. et al. Examination of mitochondria- and inflammasome-mediated mechanisms of clozapine-induced myocarditis using patient-derived iPSC cardiomyocytes. Mol Psychiatry 30, 3491–3501 (2025). https://doi.org/10.1038/s41380-025-02935-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02935-z