Abstract
Background
Subcortical ischemic demyelination is the primary cause of vascular cognitive impairment in the elderly. However, its underlying mechanisms remain elusive.
Methods
Using a bilateral common carotid artery stenosis (BACS) mouse model and an in vitro cerebellar slice model treated with low glucose-low oxygen (LGLO), we investigated a novel mechanism of vascular demyelination.
Results
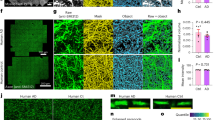
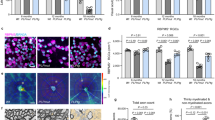
This work identified syntaphilin-mediated docking of mitochondria as the initial event preceding ischemic demyelination. This axonal insult drives paranodal retraction, myelin instability, and subsequent cognitive impairment through excessive oxidation of protein 4.1B by mitochondrial ROS. Syntaphilin knockdown reestablished the balance of mitochondrial axoplasmic transport, reduced axonal ROS burden, and consequently decreased the abnormal oxidation of protein 4.1B, an essential component that secures the Caspr1/contactin-1/NF155 complex tethered to the axonal cytoskeleton βII-Spectrin within paranodes. This ultimately protected the paranodal structure and myelin and improved cognitive function.
Conclusions
Our findings reveal a distinct pathological characteristic of ischemic demyelination and highlight the therapeutic potential of modulating axonal mitochondrial mobility to stabilize myelin structures and improve vascular cognitive impairment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data sets presented in this study are included in full whenever possible, including a display of individual data points. Data were available from the corresponding author upon reasonable request. The source data for all figures and supplementary figures are provided as a Source Data file. Supplementary information is available on MP’s website.
References
Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666.
Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–96.
Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, Tanaka R, et al. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab. 2015;35:382–91.
Fern RF, Matute C, Stys PK. White matter injury: Ischemic and nonischemic. Glia. 2014;62:1780–9.
Huang N, Li S, Xie Y, Han Q, Xu XM, Sheng ZH. Reprogramming an energetic AKT-PAK5 axis boosts axon energy supply and facilitates neuron survival and regeneration after injury and ischemia. Curr Biol. 2021;31:3098–3114.e3097.
Zhao Y, Zhang J, Zheng Y, Zhang Y, Zhang XJ, Wang H, et al. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J Neuroinflammation. 2021;18:207.
Fehmi J, Scherer SS, Willison HJ, Rinaldi S. Nodes, paranodes and neuropathies. J Neurol Neurosurg Psychiatry. 2018;89:61–71.
McKie SJ, Nicholson AS, Smith E, Fawke S, Caroe ER, Williamson JC, et al. Altered plasma membrane abundance of the sulfatide-binding protein NF155 links glycosphingolipid imbalances to demyelination. Proc Natl Acad Sci USA. 2023;120:e2218823120.
Djannatian M, Timmler S, Arends M, Luckner M, Weil MT, Alexopoulos I, et al. Two adhesive systems cooperatively regulate axon ensheathment and myelin growth in the CNS. Nat Commun. 2019;10:4794.
Dutta DJ, Woo DH, Lee PR, Pajevic S, Bukalo O, Huffman WC, et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc Natl Acad Sci USA. 2018;115:11832–7.
Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014;128:161–75.
Manso C, Querol L, Mekaouche M, Illa I, Devaux JJ. Contactin-1 IgG4 antibodies cause paranode dismantling and conduction defects. Brain. 2016;139:1700–12.
Malara M, Lutz AK, Incearap B, Bauer HF, Cursano S, Volbracht K, et al. SHANK3 deficiency leads to myelin defects in the central and peripheral nervous system. Cell Mol Life Sci. 2022;79:371.
Teigler A, Komljenovic D, Draguhn A, Gorgas K, Just WW. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum Mol Genet. 2009;18:1897–908.
Einheber S, Meng X, Rubin M, Lam I, Mohandas N, An X, et al. The 4.1B cytoskeletal protein regulates the domain organization and sheath thickness of myelinated axons. Glia. 2013;61:240–53.
Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–9.
Washida K, Hattori Y, Ihara M. Animal models of chronic cerebral hypoperfusion: from mouse to primate. Int J Mol Sci. 2019;20:6176.
Doussau F, Dupont JL, Neel D, Schneider A, Poulain B, Bossu JL. Organotypic cultures of cerebellar slices as a model to investigate demyelinating disorders. Expert. Opin. Drug. Discov. 2017;12:1011–22.
Han Q, Xie Y, Ordaz JD, Huh AJ, Huang N, Wu W, et al. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 2020;31:623–641.e628.
Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J. Neuroscience. 2007;27:10765–76.
Liebert A, Leahy M, Maniewski R. Multichannel laser-Doppler probe for blood perfusion measurements with depth discrimination. Med Biol Eng Comput. 1998;36:740–7.
Cao Q, Chen J, Zhang Z, Shu S, Qian Y, Yang L, et al. Astrocytic CXCL5 hinders microglial phagocytosis of myelin debris and aggravates white matter injury in chronic cerebral ischemia. J Neuroinflammation. 2023;20:105.
Busch JD, Fielden LF, Pfanner N, Wiedemann N. Mitochondrial protein transport: Versatility of translocases and mechanisms. Mol Cell. 2023;83:890–910.
Wan T, Zhu W, Zhao Y, Zhang X, Ye R, Zuo M, et al. Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nat Commun. 2022;13:1134.
Xie Y, Zhang X, Xu P, Zhao N, Zhao Y, Li Y, et al. Aberrant oligodendroglial LDL receptor orchestrates demyelination in chronic cerebral ischemia. J Clin Invest. 2021;131:e128114.
Niu J, Yu G, Wang X, Xia W, Wang Y, Hoi KK, et al. Oligodendroglial ring finger protein Rnf43 is an essential injury-specific regulator of oligodendrocyte maturation. Neuron. 2021;109:3104–3118.e3106.
Rodriguez J, Escobar JB, Cheung EC, Kowalik G, Russo R, Dyavanapalli J, et al. Hypothalamic oxytocin neuron activation attenuates intermittent hypoxia-induced hypertension and cardiac dysfunction in an animal model of sleep apnea. Hypertension. 2023;80:882–94.
Lubetzki C, Sol-Foulon N, Desmazières A. Nodes of Ranvier during development and repair in the CNS. Nat. Rev. Neurol. 2020;16:426–39.
Buttermore ED, Dupree JL, Cheng J, An X, Tessarollo L, Bhat MA. The cytoskeletal adaptor protein band 4.1B is required for the maintenance of paranodal axoglial septate junctions in myelinated axons. J Neurosci. 2011;31:8013–24.
Cifuentes-Diaz C, Chareyre F, Garcia M, Devaux J, Carnaud M, Levasseur G, et al. Protein 4.1B contributes to the organization of peripheral myelinated axons. PLoS ONE. 2011;6:e25043.
Li Y, Berliocchi L, Li Z, Rasmussen LJ. Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age. Aging Cell. 2024;23:e13942.
Shanmughapriya S, Langford D, Natarajaseenivasan K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res Rev. 2020;62:101128.
Mandal A, Wong HC, Pinter K, Mosqueda N, Beirl A, Lomash RM, et al. Retrograde Mitochondrial Transport Is Essential for Organelle Distribution and Health in Zebrafish Neurons. J Neurosci. 2021;41:1371–92.
Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–19.
Chen Y, Sheng ZH. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol. 2013;202:351–64.
Joshi DC, Zhang C-L, Lin T-M, Gusain A, Harris MG, Tree E, et al. Deletion of mitochondrial anchoring protects dysmyelinating shiverer: implications for progressive MS. J Neuroscience. 2015;35:5293–306.
Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, et al. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–74.
Joshi DC, Zhang CL, Babujee L, Vevea JD, August BK, Sheng ZH, et al. Inappropriate intrusion of an axonal mitochondrial anchor into dendrites causes neurodegeneration. Cell Rep. 2019;29:685–96.e685.
Ohno N, Chiang H, Mahad DJ, Kidd GJ, Liu L, Ransohoff RM, et al. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc Natl Acad Sci USA. 2014;111:9953–8.
Licht-Mayer S, Campbell GR, Canizares M, Mehta AR, Gane AB, McGill K, et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol. 2020;140:143–67.
Alrashdi B, Dawod B, Schampel A, Tacke S, Kuerten S, Marshall JS, et al. Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis. J Neuroinflammation. 2019;16:215.
Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127:294–303.
Craner MJ, Lo AC, Black JA, Waxman SG. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain. 2003;126:1552–61.
Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–40.
Weng Z, Cao C, Stepicheva NA, Chen F, Foley LM, Cao S, et al. A novel needle mouse model of vascular cognitive impairment and dementia. J Neurosci. 2023;43:7351–60.
Matute C, Domercq M, Sánchez-Gómez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–24.
Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927.
Funding
This study was supported by the Ministry of Science and Technology of China (STI2030- Major Projects 2021ZD0201806 to M.C. and 2021YFC2500100 to Q.D), National Natural Science Foundation of China (82271221 to M.C.; 82071197 to Q.D.; 82101336 to M.G.; 82201468 to YW. F.; 82373658 to YF. J).
Author information
Authors and Affiliations
Contributions
Conceptualization, MC, QD, JY and YZ; formal analysis, YF; investigation, YF, MZ, JX, SH, JL and HZ; writing—original draft preparation, MG and TY; writing—review and editing, MG, TY, YZ, QD and MC; supervision, MC; funding acquisition, MG, QD, YJ and MC.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures were performed following the Guide for the National Science Council of the People’s Republic of China, and the study was approved by the Ethics Committee of Fudan University, Shanghai, China (IRB approval number 20180972A259). This manuscript was written in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Guo, M., You, T. et al. Paranodal instability driven by axonal mitochondrial accumulation in ischemic demyelination and cognitive decline. Mol Psychiatry 30, 3502–3515 (2025). https://doi.org/10.1038/s41380-025-02936-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02936-y