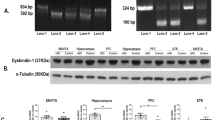
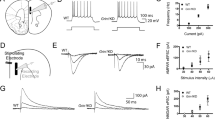
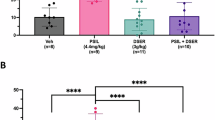
Abstract
Histidine triad nucleotide-binding protein 1 (HINT1) is related to depression. However, the underlying mechanisms and whether HINT1 is a therapeutic target for depression remain unclear. In this study, we report that loganin, an antidepressant candidate from our previous research, directly targets HINT1 to alleviate depressive-like behaviors. Overexpression of HINT1 in the hippocampus induces depressive-like behaviors. Mechanistically, HINT1 hinders sigma-1 receptor (Sigma-1R) binding to N-methyl-D-aspartate receptor (NMDAR), promotes postsynaptic density protein (PSD95) binding to NMDAR, inhibits brain derived neurotrophic factor (BDNF) signaling, and impairs synaptic plasticity. The interaction between HINT1 and NMDAR is disturbed by loganin. The antidepressant-like effects of loganin are reversed by HINT1 overexpression, Sigma-1R inhibitor and tropomyosin kinase receptor B (TrkB) inhibitor. These results not only indicate that HINT1 induces depression via impairing synaptic plasticity but also provide a candidate targeting HINT1 for depression therapy.

Zhang et al. reported that a natural compound, loganin, improves synaptic plasticity and reduces depressive-like behaviors via its direct target HINT1. Mechanistically, overexpressed HINT1 hinders NMDAR/Sigma-1R interactions and increases NMDAR/PSD95 interactions, and HINT1/NMDAR interactions are disrupted by loganin treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions. Supplementary information is available at MP’s website.
References
Herrman H, Kieling C, McGorry P, Horton R, Sargent J, Patel V. Reducing the global burden of depression: a lancet-world psychiatric association commission. Lancet. 2019;393:e42–3.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065.
Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37:134–45.
Kim JW, Suzuki K, Kavalali ET, Monteggia LM. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol Med. 2023;29:364–75.
Jha MK, Mathew SJ. Pharmacotherapies for treatment-resistant depression: how antipsychotics fit in the rapidly evolving therapeutic landscape. Am J Psychiatry. 2023;180:190–9.
Ling S, Ceban F, Lui LMW, Lee Y, Teopiz KM, Rodrigues NB, et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. 2022;36:17–30.
Liu P, Liu Z, Wang J, Ma X, Dang Y. HINT1 in neuropsychiatric diseases: a potential neuroplastic mediator. Neural Plast. 2017;2017:5181925.
Dillenburg M, Smith J, Wagner CR. The many faces of histidine triad nucleotide binding protein 1 (HINT1). ACS Pharmacol Transl Sci. 2023;6:1310–22.
Barbier E, Wang JB. Anti-depressant and anxiolytic like behaviors in PKCI/HINT1 knockout mice associated with elevated plasma corticosterone level. BMC Neurosci. 2009;10:132.
Rodríguez-Muñoz M, Cortés-Montero E, Onetti Y, Sánchez-Blázquez P, Garzón-Niño J. The σ1 Receptor and the HINT1 protein control α2δ1 binding to glutamate NMDA receptors: implications in neuropathic pain. Biomolecules. 2021;11:1681.
Pabba M, Sibille E. Sigma-1 and N-Methyl-d-Aspartate receptors: a partnership with beneficial outcomes. Mol Neuropsychiatry. 2015;1:47–51.
Cai Q, Zeng M, Wu X, Wu H, Zhan Y, Tian R, et al. CaMKIIα-driven, phosphatase-checked postsynaptic plasticity via phase separation. Cell Res. 2021;31:37–51.
Zhang F, Yan Y, Zhang J, Li L, Wang YW, Xia CY, et al. Phytochemistry, synthesis, analytical methods, pharmacological activity, and pharmacokinetics of loganin: a comprehensive review. Phytother Res. 2022;36:2272–99.
Guo Y-X, Xia C-Y, Yan Y, Han Y, Shi R, He J, et al. Loganin improves chronic unpredictable mild stress-induced depressive-like behaviors and neurochemical dysfunction. J Ethnopharmacol. 2023;308:116288.
Pan CH, Xia CY, Yan Y, Han Y, Shi R, He J, et al. Loganin ameliorates depression-like behaviors of mice via modulation of serotoninergic system. Psychopharmacology (Berl). 2021;238:3063–70.
Shi R, Han Y, Yan Y, Qiao HY, He J, Lian WW, et al. Loganin exerts sedative and hypnotic effects via modulation of the serotonergic system and GABAergic neurons. Front Pharmacol. 2019;10:409.
Zhang WK, Xu JK, He J, Qiao HY, Ye XS Application of loganin in the preparation of drugs for the prevention and treatment of depression, anxiety and other mental disorders.
Zhang WK, Xu JK, He J, Ye XS, Qiao HY, Pan XG A kind of preparation method of loganin raw material drug.
Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–20.
Su SH, Wu YF, Lin Q, Wang DP, Hai J. URB597 protects against NLRP3 inflammasome activation by inhibiting autophagy dysfunction in a rat model of chronic cerebral hypoperfusion. J Neuroinflammation. 2019;16:260.
Cheng X, Yan J, Liu Y, Wang J, Taubert S. eVITTA: a web-based visualization and inference toolbox for transcriptome analysis. Nucleic Acids Res. 2021;49:W207–15.
Zhang X, Xu H, Bi X, Hou G, Liu A, Zhao Y, et al. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways. Cell Death Dis. 2021;12:931.
Ye Z, Wang J, Fang F, Wang Y, Liu Z, Shen C, et al. Zhi-Zi-Hou-Po decoction alleviates depressive-like behavior and promotes hippocampal neurogenesis in chronic unpredictable mild stress induced mice via activating the BDNF/TrkB/CREB pathway. J Ethnopharmacol. 2024;319:117355.
Liaqat H, Parveen A, Kim SY. Antidepressive effect of natural products and their derivatives targeting BDNF-TrkB in Gut-Brain Axis. Int J Mol Sci. 2022;23:14968.
Zhou X, Chou TF, Aubol BE, Park CJ, Wolfenden R, Adams J, et al. Kinetic mechanism of human histidine triad nucleotide binding protein 1. Biochemistry. 2013;52:3588–600.
Rodríguez-Muñoz M, Sánchez-Blázquez P, Herrero-Labrador R, Martínez-Murillo R, Merlos M, Vela JM, et al. The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid Redox Signal. 2015;22:799–818.
Pabba M, Wong AY, Ahlskog N, Hristova E, Biscaro D, Nassrallah W, et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci. 2014;34:11325–38.
Yang ZJ, Carter EL, Torbey MT, Martin LJ, Koehler RC. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp Neurol. 2010;221:166–74.
Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–6.
Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74.
Castrén E, Monteggia LM. Brain-Derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90:128–36.
Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203.
Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92.
Sun Z, Jia L, Shi D, He Y, Ren Y, Yang J, et al. Deep brain stimulation improved depressive-like behaviors and hippocampal synapse deficits by activating the BDNF/mTOR signaling pathway. Behav Brain Res. 2022;419:113709.
Larsen MH, Mikkelsen JD, Hay-Schmidt A, Sandi C. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiatr Res. 2010;44:808–16.
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38:579–93.
Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–95.
Vara H, Onofri F, Benfenati F, Sassoè-Pognetto M, Giustetto M. ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc Natl Acad Sci USA. 2009;106:9872–7.
Doucet MV, Harkin A, Dev KK. The PSD-95/nNOS complex: new drugs for depression? Pharmacol Ther. 2012;133:218–29.
Compans B, Camus C, Kallergi E, Sposini S, Martineau M, Butler C, et al. NMDAR-dependent long-term depression is associated with increased short term plasticity through autophagy mediated loss of PSD-95. Nat Commun. 2021;12:2849.
Xue SG, He JG, Lu LL, Song SJ, Chen MM, Wang F, et al. Enhanced TARP-γ8-PSD-95 coupling in excitatory neurons contributes to the rapid antidepressant-like action of ketamine in male mice. Nat Commun. 2023;14:7971.
Dang YH, Liu P, Ma R, Chu Z, Liu YP, Wang JB, et al. HINT1 is involved in the behavioral abnormalities induced by social isolation rearing. Neurosci Lett. 2015;607:40–5.
Liu F, Dong YY, Lei G, Zhou Y, Liu P, Dang YH. HINT1 is involved in the chronic mild stress elicited oxidative stress and apoptosis through the PKC ε/ALDH-2/4HNE pathway in prefrontal cortex of rats. Front Behav Neurosci. 2021;15:690344.
Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, Rahmoune H, et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry. 2012;2:e87.
Zhou Y, Li SF, Deng LS, Ma YK, Lei G, Dang YH. HINT1 deficiency in aged mice reduces anxiety-like and depression-like behaviours and enhances cognitive performances. Exp Gerontol. 2022;159:111683.
Sun L, Liu P, Liu F, Zhou Y, Chu Z, Li Y, et al. Effects of Hint1 deficiency on emotional-like behaviors in mice under chronic immobilization stress. Brain Behav. 2017;7:e00831.
Jackson KJ, Wang JB, Barbier E, Chen X, Damaj MI. Acute behavioral effects of nicotine in male and female HINT1 knockout mice. Genes Brain Behav. 2012;11:993–1000.
Tian M, Stroebel D, Piot L, David M, Ye S, Paoletti P. GluN2A and GluN2B NMDA receptors use distinct allosteric routes. Nat Commun. 2021;12:4709.
Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–21.
Gao M, Wu Y, Yang L, Chen F, Li L, Li Q, et al. Anti-depressant-like effect of fermented Gastrodia elata Bl. by regulating monoamine levels and BDNF/NMDAR pathways in mice. J Ethnopharmacol. 2023;301:115832.
Liu B, Du Y, Xu C, Liu Q, Zhang L. Antidepressant effects of repeated s-ketamine administration as NMDAR antagonist: involvement of CaMKIIα and mTOR signaling in the hippocampus of CUMS mice. Brain Res. 2023;1811:148375.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Rodríguez-Muñoz M, Cortés-Montero E, Pozo-Rodrigálvarez A, Sánchez-Blázquez P, Garzón-Niño J. The ON:OFF switch, σ1R-HINT1 protein, controls GPCR-NMDA receptor cross-regulation: implications in neurological disorders. Oncotarget. 2015;6:35458–77.
Valkov E, Sharpe T, Marsh M, Greive S, Hyvönen M. Targeting protein-protein interactions and fragment-based drug discovery. Top Curr Chem. 2012;317:145–79.
Shi X, Zhou XZ, Chen G, Luo WF, Zhou C, He TJ, et al. Targeting the postsynaptic scaffolding protein PSD-95 enhances BDNF signaling to mitigate depression-like behaviors in mice. Sci Signal. 2024;17:eadn4556.
Luo CX, Lin YH, Qian XD, Tang Y, Zhou HH, Jin X, et al. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J Neurosci. 2014;34:13535–48.
Fishback JA, Robson MJ, Xu Y-T, Matsumoto RR. Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol Ther. 2010;127:271–82.
Yang K, Sun T. The roles of chaperone proteins, sigma receptors, in Parkinson’s disease (PD) and major depressive disorder (MDD). Front Pharmacol. 2019;10:528.
Vavers E, Zvejniece L, Maurice T, Dambrova M. Allosteric modulators of sigma-1 receptor: a review. Front Pharmacol. 2019;10:223.
Liu X, Qu C, Shi S, Ye T, Wang L, Liu S, et al. The reversal effect of Sigma-1 receptor (S1R) agonist, SA4503, on atrial fibrillation after depression and its underlying mechanism. Front Physiol. 2019;10:1346.
Volz H-P, Stoll K. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37:214–20.
Di T, Zhang S, Hong J, Zhang T, Chen L. Hyperactivity of hypothalamic-pituitary-adrenal axis due to dysfunction of the hypothalamic glucocorticoid receptor in sigma-1 receptor knockout mice. Front Mol Neurosci. 2017;10:287.
Fujimoto M, Hayashi T, Urfer R, Mita S, Su T-P. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–9.
Ji LL, Peng JB, Fu CH, Tong L, Wang ZY. Sigma-1 receptor activation ameliorates anxiety-like behavior through NR2A-CREB-BDNF signaling pathway in a rat model submitted to single-prolonged stress. Mol Med Rep. 2017;16:4987–93.
Acknowledgements
This work was supported by National High Level & Elite Medical Professionals Project of China-Japan Friendship Hospital (2024-NHLHCRF-JBGS-WZ-07, 2023-NHLHCRF-CXYW-01, ZRJY2021-QM16, ZRJY2024-BJ01), National Natural Science Foundation of China (82474100, 82273815, 82273809, 82073731), and Beijing Key Laboratory of Mental Disorders (2022JSJB03).
Author information
Authors and Affiliations
Contributions
GY Zuo and YX Guo. performed the cellular and animal experiments. GY Zuo, MN Wang and YM Wang. wrote the manuscript. CY Xia and HL Xiang. performed bioinformatics analysis. GY Zuo, YM Wang and Y Han assisted with animal experiments and prepared mouse models. CY Xia, GY Zuo, YM Wang and MN Wang analyzed the data. WK Zhang, JK Xu., J He and, CY Xia provided the idea and designed the experiments. WK Zhang, JK Xu, J He, CY Xia and YC Cheng reviewed and checked the final paper. All the authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, C., Zuo, G., Wang, M. et al. Targeting HINT1 to improve synaptic plasticity: toward loganin as a new antidepressant strategy. Mol Psychiatry 30, 3695–3707 (2025). https://doi.org/10.1038/s41380-025-02959-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02959-5