Abstract
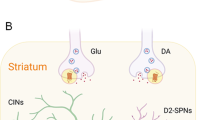
The gene CELSR3 (Cadherin EGF LAG Seven‐pass-G‐type Receptor 3) has been recently recognized as a high-confidence risk factor for Tourette syndrome (TS). Additionally, Celsr3 mutant mice have been reported to exhibit TS-related behaviors and increased dopamine release in the striatum. Building on these findings, we further characterized the neurobehavioral and molecular profile of Celsr3 mutant mice to understand better the biological mechanisms connecting the deficiency of this gene and TS-related phenotypes. Our analyses confirmed that Celsr3 mutant mice displayed grooming stereotypies and tic-like jerks, as well as sensorimotor gating deficits, which were opposed by TS therapies. Spatial transcriptomic analyses revealed widespread extracellular matrix abnormalities in the striatum of Celsr3 mutants. Single-nucleus transcriptomics also showed significant upregulation of the Drd3 gene, encoding the dopamine D3 receptor, in striosomal D1-positive neurons. In situ hybridization and immunofluorescence confirmed dysregulated D3 receptor expression, with lower levels in presynaptic striatal fibers and higher levels in striatal D1-positive neurons. Activating and blocking D3 receptors amplified or decreased tic-like jerks and stereotypies in Celsr3-deficient mice, respectively. These findings suggest that modifications of D3 receptor distribution contribute to the tic-like responses associated with Celsr3 deficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The data generated for sn-RNA seq are available on Gene Expression Omnibus (GSE249902 and GSE280709).
References
Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry. 2010;197:36–44.
Cooper C, Livingston G. Psychological morbidity and caregiver burden in parents of children with Tourette’s disorder and psychiatric comorbidity. J Am Acad Child Adolesc Psychiatry. 2003;42:1370–5.
Eddy CM, Rizzo R, Gulisano M, Agodi A, Barchitta M, Calì P, et al. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol. 2011;258:291–301.
Eapen V, Snedden C, Črnčec R, Pick A, Sachdev P. Tourette syndrome, co-morbidities and quality of life. Aust N Z J Psychiatry. 2016;50:82–93.
Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, et al. Exploring the impact of chronic tic disorders on youth: results from the Tourette syndrome impact survey. Child Psychiatry Hum Dev. 2011;42:219–42.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth Edition. Washington, DC; American Psychiatric Association: 2013.
Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord Off J Mov Disord Soc. 2015;30:221–8.
Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436–47.
Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19.
Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. 2009;67:497–501.
Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21:451–7.
Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–33.
Martino D, Ganos C, Pringsheim TM. Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. 2017;134:1461–90.
Rizzo R, Gulisano M, Martino D, Robertson MM. Gilles de la Tourette syndrome, depression, depressive illness, and correlates in a child and adolescent population. J Child Adolesc Psychopharmacol. 2017;27:243–9.
Billnitzer A, Jankovic J. Current management of tics and Tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. 2020;17:1681–93.
Kompoliti K, Goetz CG, Morrissey M, Leurgans S. Gilles de la Tourette syndrome: Patient’s knowledge and concern of adverse effects. Mov Disord. 2006;21:248–52.
Jerrell JM, McIntyre RS. Adverse events in children and adolescents treated with antipsychotic medications. Hum Psychopharmacol Clin Exp. 2008;23:283–90.
Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci Off J Soc Neurosci. 2011;31:12387–95.
Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–82.
Maia TV, Conceição VA. Dopaminergic disturbances in Tourette syndrome: an integrative account. Biol Psychiatry. 2018;84:332–44.
Steeves TDL, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, et al. Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann Neurol. 2010;67:170–81.
Singer HS, Szymanski S, Giuliano J, Yokoi F, Dogan AS, Brasic JR, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159:1329–36.
Kalanithi PSA, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102:13307–12.
Kataoka Y, Kalanithi PSA, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91.
Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in tourette syndrome. Biol Psychiatry. 2016;79:372–82.
Willsey AJ, Fernandez TV, Yu D, King RA, Dietrich A, Xing J, et al. De novo coding variants are strongly associated with Tourette disorder. Neuron. 2017;94:486–9.e9.
Wang S, Mandell JD, Kumar Y, Sun N, Morris MT, Arbelaez J, et al. De novo sequence and copy number variants are strongly associated with Tourette disorder and implicate cell polarity in pathogenesis. Cell Rep. 2018;24:3441–54.e12.
Zhao X, Wang S, Hao J, Zhu P, Zhang X, Wu M. A whole-exome sequencing study of Tourette disorder in a chinese population. DNA Cell Biol. 2020;39:63–X8.
Goffinet AM, Tissir F. Seven pass cadherins CELSR1-3. Semin Cell Dev Biol. 2017;69:102–10.
Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat Neurosci. 2005;8:451–7.
Wang X, Zhang D, Xu Z, Ma M, Wang W, Li L, et al. Understanding cadherin EGF LAG seven‐pass G‐type receptors. J Neurochem. 2014;131:699–711.
Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, et al. Wnt/Planar cell polarity signaling controls the anterior–posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30:16053–64.
Jia Z, Guo Y, Tang Y, Xu Q, Li B, Wu Q. Regulation of the protocadherin Celsr3 gene and its role in globus pallidus development and connectivity. Mol Cell Biol. 2014;34:3895–10.
Qu Y, Huang Y, Feng J, Alvarez-Bolado G, Grove EA, Yang Y, et al. Genetic evidence that Celsr3 and Celsr2, together with Fzd3, regulate forebrain wiring in a Vangl -independent manner. Proc Natl Acad Sci. 2014;111:E2996–3004.
Feng J, Xian Q, Guan T, Hu J, Wang M, Huang Y, et al. Celsr3 and Fzd3 organize a pioneer neuron scaffold to steer growing thalamocortical axons. Cereb Cortex. 2016;26:3323–34.
Nasello C, Poppi LA, Wu J, Kowalski TF, Thackray JK, Wang R, et al. Human mutations in high-confidence Tourette disorder genes affect sensorimotor behavior, reward learning, and striatal dopamine in mice. Proc Natl Acad Sci. 2024;121:e2307156121.
Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41.
Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50:578–85.
Wu S, Ying G, Wu Q, Capecchi MR. A protocol for constructing gene targeting vectors: generating knockout mice for the cadherin family and beyond. Nat Protoc. 2008;3:1056–76.
Ying G, Wu S, Hou R, Huang W, Capecchi MR, Wu Q. The protocadherin gene Celsr3 is required for interneuron migration in the mouse forebrain. Mol Cell Biol. 2009;29:3045–61.
Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, Fowler SC, et al. The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol. 2016;173:2111–21.
Cadeddu R, Van Zandt M, Santovito LS, Odeh K, Anderson CJ, Flanagan D, et al. Prefrontal allopregnanolone mediates the adverse effects of acute stress in a mouse model of tic pathophysiology. Neuropsychopharmacology. 2023;48:1288–99.
Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. Ecopipam for Tourette syndrome: a randomized trial. Pediatrics. 2023;151:e2022059574.
Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord Off J Mov Disord Soc. 2011;26:2146–7.
Mosher LJ, Godar SC, Nelson M, Fowler SC, Pinna G, Bortolato M. Allopregnanolone mediates the exacerbation of Tourette-like responses by acute stress in mouse models. Sci Rep. 2017;7:3348.
Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, et al. Genetic-based dissection unveils the inputs and outputs of striatal patch and matrix compartments. Neuron. 2016;91:1069–84.
Märtin A, Calvigioni D, Tzortzi O, Fuzik J, Wärnberg E, Meletis K. A spatiomolecular map of the striatum. Cell Rep. 2019;29:4320–33.e5.
Nordstrom EJ, Burton FH. A transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder circuitry. Mol Psychiatry. 2002;7:617–25. 524
Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev. 2017;76:123–33.
Mosher LJ, Cadeddu R, Yen S, Staudinger JL, Traccis F, Fowler SC, et al. Allopregnanolone is required for prepulse inhibition deficits induced by D1 dopamine receptor activation. Psychoneuroendocrinology. 2019;108:53–61.
Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment ofTourette syndrome. Drugs Today. 2014;50:159.
Malech H, Root R, Gallin J. Structural analysis of human neutrophil migration: centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977;75:666–93.
Gallin JI, Rosenthal AS. The regulatory role of divalent cations in human granulocyte chemotaxis. J Cell Biol. 1974;62:594–609.
Shima Y, Kawaguchi S, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, et al. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat Neurosci. 2007;10:963–9.
Tissir F, Goffinet AM. Atypical cadherins Celsr1–3 and planar cell polarity in vertebrates. Prog Mol Biol Transl Sci. 2013;116:193–214.
Owen CA, Campbell EJ. Neutrophil proteinases and matrix degradation. The cellbiology of pericellular proteolysis. Semin Cell Biol. 1995;6:367–6.
Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18:721–8.
Liu S, Yu X, Xu Q, Cui J, Yi M, Zhang X, et al. Support of positive association in family-based genetic analysis between COL27A1 and Tourette syndrome. Sci Rep. 2015;5:12687.
Fox MA. Novel roles for collagens in wiring the vertebrate nervous system. Curr Opin Cell Biol. 2008;20:508–13.
Wareham LK, Baratta RO, Del Buono BJ, Schlumpf E, Calkins DJ. Collagen in the central nervous system: contributions to neurodegeneration and promise as a therapeutic target. Mol Neurodegener. 2024;19:11.
Gregorio I, Mereu M, Contarini G, Bello L, Semplicini C, Burgio F, et al. Collagen VI deficiency causes behavioral abnormalities and cortical dopaminergic dysfunction. Dis Model Mech. 2022;15:dmm049481.
Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–8.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8.
Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–41.
Martino D, Zis P, Buttiglione M. The role of immune mechanisms in Tourette syndrome. Brain Res. 2015;1617:126–43.
Frick L, Pittenger C. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J Immunol Res. 2016;2016:1–8.
Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51.
Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci. 1992;89:8155–9.
Mahmoudi S, Lévesque D, Blanchet PJ. Upregulation of dopamine D3, not D2, receptors correlates with tardive dyskinesia in a primate model. Mov Disord. 2014;29:1125–33.
Cote SR, Chitravanshi VC, Bleickardt C, Sapru HN, Kuzhikandathil EV. Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behav Brain Res. 2014;263:46–50.
Solís O, Garcia-Montes JR, González-Granillo A, Xu M, Moratalla R. Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb Cortex. 2015;27:435–46.
Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, et al. Identification of dopamine D1–D3 receptor heteromers. J Biol Chem. 2008;283:26016–25.
Godar SC, Mosher LJ, Scheggi S, Devoto P, Moench KM, Strathman HJ, et al. Gene-environment interactions in antisocial behavior are mediated by early-life 5-HT2A receptor activation. Neuropharmacology. 2019;159:107513.
Piras IS, Braccagni G, Huentelman MJ, Bortolato M. A preliminary transcriptomic analysis of the orbitofrontal cortex of antisocial individuals. CNS Neurosci Ther. 2023;29:3173–82.
Acknowledgements
We acknowledge the Huntsman Cancer Institute High-throughput Genomics and Bioinformatics Center, as well as the HSC Cell Imaging Core, for enabling sh-RNA sequencing and IF microscopy analyses. We thank Karen Odeh, Job Huxford, Elise Vandamme, and Easton Van Luik for their technical assistance with animal testing. We thank Anton Classen for his invaluable support in image acquisition and analysis.
Funding
This study was partially supported by the NIH grant R21 NS125519 (to MB).
Author information
Authors and Affiliations
Contributions
Conceptualization: MB; Methodology: RC, CB, GB, TM, IP, CA, MC, MH, and PM; Formal analysis: RC, CB, GB, TM, IP, CA, MC, MH, PM, and MB; Writing: MB produced the initial manuscript, and all others reviewed and commented on the manuscript; Supervision: MB; Funding Acquisition: MB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Ethics approval
All methods in this study were conducted in compliance with relevant guidelines and regulations. Animal procedures were carried out in accordance with these standards and were approved by the Institutional Animal Care and Use Committees (IACUC) at the respective institutions under protocol numbers 21-11004 (University of Utah) and IACUC202400000556 (University of Florida).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cadeddu, R., Branca, C., Braccagni, G. et al. Tic-related behaviors in Celsr3 mutant mice are contributed by alterations of striatal D3 dopamine receptors. Mol Psychiatry 30, 3912–3924 (2025). https://doi.org/10.1038/s41380-025-02970-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02970-w