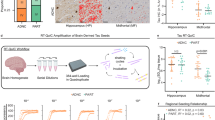
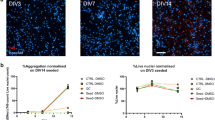
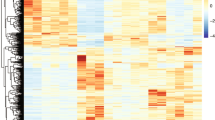
Abstract
Heterogeneity in progression of clinical dementia obstructs the general therapeutic potential of current treatments for Alzheimer’s disease (AD). Though the mechanisms of this heterogeneity remain unclear, the characterization of bioactive tau species and factors that regulate their seeding behavior might give valuable insight as pathological tau is well correlated with cognitive impairment. Here, we conducted an innovative investigation into the molecular basis of widespread, connectivity-based tau propagation that begins in the inferior temporal gyrus (ITG) and spreads to neocortical areas such as the prefrontal cortex (PFC). Biochemical analysis of human postmortem ITG and PFC tissues revealed individual variability in tau seeding, which correlated with cognitive decline, particularly in the ITG, a region known for promoting accelerated tau propagation. Notably, this study presents the first evidence that site-specific phosphorylation and isoform composition of both aggregation-prone high-molecular-weight (HMW) tau and the relatively unexplored, yet potentially crucial in AD progression low-molecular-weight (LMW) tau significantly contribute to tau propagation and cognitive decline. Our findings underscore the importance of comprehensively considering diverse tau forms including both HMW and LMW tau species in understanding AD progression. Additionally, these results are the first to identify distinct morphological strains within the AD brain associated with differing seeding propensity, potentially enabling patient stratification based on their tau profile. Furthermore, RNA-seq analyses of gene expression patterns in the ITG revealed molecular heterogeneity associated with tau seeding potential. Patients with higher levels of seed-competent tau displayed greater impairments in synaptic and neural plasticity, and increased neuroinflammation. This multidisciplinary study offers novel insights into various molecular mechanisms driving AD progression, suggesting potential molecular targets for early intervention and improved patient subtyping, which is critical for developing precision medicine approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data supporting the findings of this study are available in the main text and supplementary information files. All other data generated in the study are available from the corresponding author on request. The RNA-seq datasets generated for the current study have been deposited in GEO under the accession ID: GSE282910.
Code availability
All codes used in this study will be made available upon request from the corresponding author.
References
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2022;18:700–89.
Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nature Neuroscience. 1998;1:355–8.
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica. 2006;112:389–404.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59.
Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cerebral Cortex. 1994;4:138–50.
Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51.
Lee WJ, Brown JA, Kim HR, La Joie R, Cho H, Lyoo CH, et al. Regional Aβ-tau interactions promote onset and acceleration of Alzheimer’s disease tau spreading. Neuron. 2022;110:1932–1943.e5.
Wilkosz PA, Seltman HJ, Devlin B, Weamer EA, Lopez OL, DeKosky ST, et al. Trajectories of cognitive decline in Alzheimer’s disease. International Psychogeriatrics. 2010;22:281–90.
Komarova NL, Thalhauser CJ. High degree of heterogeneity in Alzheimer’s disease progression patterns. PLoS Computational Biology. 2011;7:e1002251.
Thalhauser CJ, Komarova NL. Alzheimer’s disease: rapid and slow progression. Journal of the Royal Society, Interface / the Royal Society. 2012;9:119–26.
Marques-Coelho D, Iohan LDCC, Melo de Farias AR, Brainbank Neuro–CEB Neuropathology Network, Lambert JC, Costa MR. Differential transcript usage unravels gene expression alterations in Alzheimer’s disease human brains. NPJ Aging and Mechanisms of Disease. 2021;7:2.
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration. 2019;14:32.
Fu H, Possenti A, Freer R, Nakano Y, Hernandez Villegas NC, Tang M, et al. A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nature Neuroscience. 2019;22:47–56.
Roussarie JP, Yao V, Rodriguez-Rodriguez P, Oughtred R, Rust J, Plautz Z, et al. Selective neuronal vulnerability in Alzheimer’s disease: a network-based analysis. Neuron. 2020;107:821–835.e12.
Rasmussen J, Ewing AD, Bodea LG, Bodea GO, Gearing M, Faulkner GJ. An early proinflammatory transcriptional response to tau pathology is age-specific and foreshadows reduced tau burden. Brain Pathology. 2022;32:e13018.
Devi G, Scheltens P. Heterogeneity of Alzheimer’s disease: consequence for drug trials? Alzheimer's Research & Therapy. 2018;10:122.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. The New England Journal of Medicine. 2021;384:1691–704.
Dujardin S, Bégard S, Caillierez R, Lachaud C, Carrier S, Lieger S, et al. Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathologica Communications. 2018;6:132.
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nature Medicine. 2020;26:1256–63.
Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, et al. Proteopathic tau seeding predicts tauopathy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4376–E4385.
Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–88.
Briner A, Götz J, Polanco JC. Fyn kinase controls tau aggregation in vivo. Cell Rep. 2020;32:108045.
McKenzie AT, Wang M, Hauberg ME, Fullard JF, Kozlenkov A, Keenan A, et al. Brain cell type specific gene expression and co-expression network architectures. Scientific Reports. 2018;8:8868.
Koopmans F, van Nierop P, Andres-Alonso M, Byrnes A, Cijsouw T, Coba MP, et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron. 2019;103:217–234.e4.
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists. Nucleic Acids Research. 2019;47:W191–W198.
Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE. 2010;5:e13984.
Lachmann A, Giorgi FM, Lopez G, Califano A. ARACNe-AP: gene network reverse engineering through adaptive partitioning inference of mutual information. Bioinformatics. 2016;32:2233–5.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. Cytohubba: identifying hub objects and sub-networks from complex interactome. BMC Systems Biology. 2014;8(Suppl 4):S11.
Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47:72–89.
Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. The Journal of Biological Chemistry. 2016;291:12445–66.
DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017;9:eaag0481.
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–26.
Zhong Q, Congdon EE, Nagaraja HN, Kuret J. Tau isoform composition influences rate and extent of filament formation. The Journal of Biological Chemistry. 2012;287:20711–9.
Dregni AJ, Duan P, Xu H, Changolkar L, El Mammeri N, Lee VM, et al. Fluent molecular mixing of Tau isoforms in Alzheimer’s disease neurofibrillary tangles. Nature Communications. 2022;13:2967.
Takeda S, Wegmann S, Cho H, DeVos SL, Commins C, Roe AD, et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nature Communications. 2015;6:8490.
Tracy TE, Sohn PD, Minami SS, Wang C, Min SW, Li Y, et al. Acetylated Tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron. 2016;90:245–60.
Colom-Cadena M, Davies C, Sirisi S, Lee JE, Simzer EM, Tzioras M, et al. Synaptic oligomeric tau in Alzheimer’s disease - A potential culprit in the spread of tau pathology through the brain. Neuron. 2023;111:2170–2183.e6.
Südhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987;238:1142–4.
Kim E, Sheng M. PDZ domain proteins of synapses. Nature Reviews Neuroscience. 2004;5:771–81.
De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–15.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207.
Maiese K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regeneration Research. 2021;16:448–55.
Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cellular and Molecular Life Sciences. 2020;77:651–63.
Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature Neuroscience. 2009;12:1097–105.
Richetin K, Moulis M, Millet A, Arràzola MS, Andraini T, Hua J, et al. Amplifying mitochondrial function rescues adult neurogenesis in a mouse model of Alzheimer’s disease. Neurobiology of Disease. 2017;102:113–24.
Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202.
Wilczynska KM, Singh SK, Adams B, Bryan L, Rao RR, Valerie K, et al. Nuclear factor I isoforms regulate gene expression during the differentiation of human neural progenitors to astrocytes. Stem Cells. 2009;27:1173–81.
Laug D, Huang TW, Huerta NAB, Huang AY, Sardar D, Ortiz-Guzman J, et al. Nuclear factor I-A regulates diverse reactive astrocyte responses after CNS injury. The Journal of Clinical Investigation. 2019;129:4408–18.
Tchieu J, Calder EL, Guttikonda SR, Gutzwiller EM, Aromolaran KA, Steinbeck JA, et al. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nature Biotechnology. 2019;37:267–75.
Dai DL, Li M, Lee EB. Human Alzheimer’s disease reactive astrocytes exhibit a loss of homeostastic gene expression. Acta Neuropathologica Communications. 2023;11:127.
Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, et al. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Molecular and Cellular Biology. 1996;16:5335–45.
Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577:566–71.
Griffin EN, Jucius T, Sim SE, Harris BS, Heinz S, Ackerman SL. RREB1 regulates neuronal proteostasis and the microtubule network. Science Advances. 2024;10:eadh3929.
Boulton SJ, Jackson SP. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Research. 1996;24:4639–48.
Bertinato J, Schild-Poulter C, Haché RJ. Nuclear localization of Ku antigen is promoted independently by basic motifs in the Ku70 and Ku80 subunits. Journal of Cell Science. 2001;114:89–99.
Fink LS, Lerner CA, Torres PF, Sell C. Ku80 facilitates chromatin binding of the telomere binding protein, TRF2. Cell Cycle. 2010;9:3798–806.
Butler AA, Johnston DR, Kaur S, Lubin FD. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Science signaling. 2019;12:eaaw9277.
Wang Z, Zhao Y, Xu N, Zhang S, Wang S, Mao Y, et al. NEAT1 regulates neuroglial cell mediating Aβ clearance via the epigenetic regulation of endocytosis-related genes expression. Cellular and Molecular Life Sciences. 2019;76:3005–18.
Chanda K, Jana NR, Mukhopadhyay D. Receptor tyrosine kinase ROR1 ameliorates Aβ1-42 induced cytoskeletal instability and is regulated by the miR146a-NEAT1 nexus in Alzheimer’s disease. Scientific Reports. 2021;11:19254.
Ercan-Herbst E, Ehrig J, Schöndorf DC, Behrendt A, Klaus B, Gomez Ramos B, et al. A post-translational modification signature defines changes in soluble tau correlating with oligomerization in early stage Alzheimer’s disease brain. Acta Neuropathologica Communications. 2019;7:192.
Guan PP, Wang P. The involvement of post-translational modifications in regulating the development and progression of Alzheimer’s disease. Molecular Neurobiology. 2023;60:3617–32.
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9535–40.
Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathologica. 2002;103:26–35.
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–99.
Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 2020;183:1699–1713.e13.
Mondragon-Rodríguez S, Perry G, Luna-Muñoz J, Acevedo-Aquino MC, Williams S. Phosphorylation of tau protein at sites Ser(396–404) is one of the earliest events in Alzheimer’s disease and down syndrome. Neuropathology and Applied Neurobiology. 2014;40:121–35.
Jeganathan S, Hascher A, Chinnathambi S, Biernat J, Mandelkow EM, Mandelkow E. Proline-directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of Tau and generates a pathological (MC-1) conformation. The Journal of Biological Chemistry. 2008;283:32066–76.
Helboe L, Rosenqvist N, Volbracht C, Pedersen LØ, Pedersen JT, Christensen S, et al. Highly specific and sensitive target binding by the humanized pS396-Tau antibody hC10.2 across a wide spectrum of Alzheimer’s disease and primary tauopathy postmortem brains. Journal of Alzheimer's disease : JAD. 2022;88:207–28.
Moloney CM, Lowe VJ, Murray ME. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: a clinicopathologic perspective for biomarker research. Alzheimer's & Dementia : Diagnosis, Assessment & Disease Monitoring. 2021;17:1554–74.
Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130144.
Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nature Medicine. 2020;26:379–86.
Karikari TK, Emeršič A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimer's & Dementia : Diagnosis, Assessment & Disease Monitoring. 2021;17:755–67.
Luna-Muñoz J, García-Sierra F, Falcón V, Menéndez I, Chávez-Macías L, Mena R. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. Journal of Alzheimer's disease : JAD. 2005;8:29–41.
Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006247.
Santa-Maria I, Varghese M, Ksiezak-Reding H, Dzhun A, Wang J, Pasinetti GM. Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. The Journal of Biological Chemistry. 2012;287:20522–33.
Barthélemy NR, Bateman RJ, Hirtz C, Marin P, Becher F, Sato C, et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimer's Research & Therapy. 2020;12:26.
Tissot C, Therriault J, Kunach P, Benedet LA, Pascoal TA, Ashton NJ, et al. Comparing tau status determined via plasma pTau181, pTau231 and [18F]MK6240 tau-PET. EBioMedicine. 2022;76:103837.
Ashton NJ, Benedet AL, Pascoal TA, Karikari TK, Lantero-Rodriguez J, Brum WS, et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine. 2022;76:103836.
Saroja SR, Sharma A, Hof PR, Pereira AC. Differential expression of tau species and the association with cognitive decline and synaptic loss in Alzheimer’s disease. Alzheimer's & Dementia : Diagnosis, Assessment & Disease Monitoring. 2022;18:1602–15.
Saunders TS, Pozzolo FE, Heslegrave A, King D, McGeachan RI, Spires-Jones MP, et al. Predictive blood biomarkers and brain changes associated with age-related cognitive decline. Brain Commun. 2023;5:fcad113.
Mirbaha H, Chen D, Morazova OA, Ruff KM, Sharma AM, Liu X, et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife. 2018;7:e36584.
Mirbaha H, Chen D, Mullapudi V, Terpack SJ, White CL 3rd, et al. Seed-competent tau monomer initiates pathology in a tauopathy mouse model. The Journal of Biological Chemistry. 2022;298:102163.
Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10252–7.
Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Frontiers in Neurology. 2013;4:122.
Quinn JP, Corbett NJ, Kellett KAB, Hooper NM. Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. Journal of Alzheimer's disease : JAD. 2018;63:13–33.
Ozcelik S, Sprenger F, Skachokova Z, Fraser G, Abramowski D, Clavaguera F, et al. Co-expression of truncated and full-length tau induces severe neurotoxicity. Molecular Psychiatry. 2016;21:1790–8.
Zhang Z, Song M, Liu X, Kang SS, Kwon IS, Duong DM, et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nature Medicine. 2014;20:1254–62.
Matsumoto SE, Motoi Y, Ishiguro K, Tabira T, Kametani F, Hasegawa M, et al. The twenty-four KDa C-terminal tau fragment increases with aging in tauopathy mice: implications of prion-like properties. Human Molecular Genetics. 2015;24:6403–16.
Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of tau fibrillization. FEBS Letters. 2006;580:211–5.
Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Letters. 2002;530:209–14.
Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nature Chemical Biology. 2008;4:483–90.
Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Molecular Cell. 2000;6:873–83.
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Seitelberger F, et al. Tau and ubiquitin immunoreactivity at different stages of formation of Alzheimer neurofibrillary tangles. Progress in Clinical and Biological Research. 1989;317:837–48.
Bancher C, Grundke-Iqbal I, Iqbal K, Fried VA, Smith HT, Wisniewski HM. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Research. 1991;539:11–8.
Liu C, Götz J. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS ONE. 2013;8:e84849.
Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT, et al. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E8187–E8196.
Tuerde D, Kimura T, Miyasaka T, Furusawa K, Shimozawa A, Hasegawa M, et al. Isoform-independent and -dependent phosphorylation of microtubule-associated protein tau in mouse brain during postnatal development. The Journal of Biological Chemistry. 2018;293:1781–93.
Cherry JD, Esnault CD, Baucom ZH, Tripodis Y, Huber BR, Alvarez VE, et al. Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer’s disease. Acta Neuropathologica Communications. 2021;9:86.
Marcatti M, Fracassi A, Montalbano M, Natarajan C, Krishnan B, Kayed R, et al. Aβ/tau oligomer interplay at human synapses supports shifting therapeutic targets for Alzheimer’s disease. Cellular and Molecular Life Sciences. 2022;79:222.
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature Reviews Neurology. 2021;17:157–72.
Tzioras M, McGeachan RI, Durrant CS, Spires-Jones TL. Synaptic degeneration in Alzheimer disease. Nature Reviews Neurology. 2023;19:19–38.
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neuroscience Research. 2014;79:1–12.
Barisano G, Montagne A, Kisler K, Schneider JA, Wardlaw JM, Zlokovic BV. Blood-brain barrier link to human cognitive impairment and Alzheimer’s disease. Nature Cardiovascular Research. 2022;1:108–15.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2016;17:5–21.
Avila J, Gómez-Ramos A, Bolós M. AD genetic risk factors and tau spreading. Frontiers in Aging Neuroscience. 2015;7:99.
Kaufman SK, Thomas TL, Del Tredici K, Braak H, Diamond MI. Characterization of tau prion seeding activity and strains from formaldehyde-fixed tissue. Acta Neuropathologica Communications. 2017;5:41.
Furman JL, Vaquer-Alicea J, White CL 3rd, Cairns NJ, Nelson PT, Diamond MI. Widespread tau seeding activity at early Braak stages. Acta Neuropathologica. 2017;133:91–100.
Acknowledgements
We thank the Alzheimer’s Disease Research Center (ADRC) at the Icahn School of Medicine at Mount Sinai, Mount Sinai Brain Bank (NIH NeuroBioBank), especially Dr. Vahram Haroutunian (Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA) and Banner Sun Health Research Institute, especially Dr. Thomas Beach and Dr. Geidy E. Serrano (Banner Sun Health Research Institute, Sun City, Arizona, USA) for providing human brain samples, and microscopy core at Icahn School of Medicine at Mount Sinai, especially Dr. Shilpa Dilip Kumar and Glenn Doherty. We thank Dr. Patrick Hof (Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, USA), Dr. Minghui Wang (Department of Genetics & Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, USA), Drs. Banshi Nath and Kaitlin Murtha (Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, USA) for their insightful comments and feedback. This work was supported by NIH Grants R01 AG063819 and R01 AG064020 (to A.C.P.), the DANA Foundation (to A.C.P.); the Alzheimer’s Association (to A.C.P.); the Sanford J Grossman Charitable Trust (to A.C.P.); Brian and Tania Higgins Charitable Foundation (to A.C.P.); the Carolyn and Eugene Mercy Research Gift (to A.C.P.); the Karen Strauss Cook Research Scholar Award (to A.C.P.); the Alzheimer’s New Jersey (to A.C.P); the Robert J. and Claire Pasarow Foundation (to A.C.P.); and NIH/NIA P30AG066514 Alzheimer’s Disease Research Center (to M.S.).
Author information
Authors and Affiliations
Contributions
AC and ACP designed the research. AC and MF performed experiments. AC, AR, ACP, LS, MF, DB and JHS analyzed experiments. MS and CZ provided intellectual input while selecting subjects for the study. ACP and LS contributed reagents/analytic tools. AC wrote the manuscript. AC, ACP, LS, AR, MF, DB and CZ edited the manuscript. All authors provided feedback on the manuscript draft.
Corresponding author
Ethics declarations
Competing interests
ACP has patents unrelated to this work licensed to Neurobiopharma, LLC, serves on the scientific advisory board of Sinaptica Therapeutics and has served as a consultant to Eisai and Quanterix.
Ethics approval and consent to participate
For human brain tissue, written informed consent for post-mortem brain donation was obtained from the families of donors through the brain bank. Prior to their transfer to the Icahn School of Medicine at Mount Sinai, all samples were de-identified, thereby exempting them from the oversight of the Institutional Review Board (IRB).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chongtham, A., Ramakrishnan, A., Farinas, M. et al. Neocortical tau propagation is a mediator of clinical heterogeneity in Alzheimer’s disease. Mol Psychiatry 30, 4194–4213 (2025). https://doi.org/10.1038/s41380-025-02998-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-02998-y