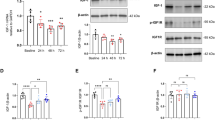
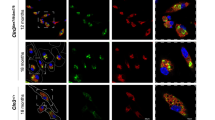
Abstract
Perioperative neurocognitive disorders (PND), including postoperative delirium (POD), delayed neurocognitive recovery (dNCR) and postoperative neurocognitive disorder (PNCD), affect up to 10% of surgical patients older than 60 years, and currently there are no effective therapies to prevent PND. The gut microbiota is linked to PND through the gut-brain axis, promoting neuroinflammation via activation and proliferation of microglia and astrocytes in the central nervous system (CNS). In this study, we show that perioperative use of ceftriaxone, a long-acting β-lactam antibiotic, can prevent the development of PND in elderly surgical patients. This effect is associated with reduced serum complement C3 levels and increased levels of platelet factor 4 (PF4). Using an aged mouse model of PND, we found that C3/C3aR axis mediated the interaction of astroglia and microglia during the early stages of neuroinflammation. Genetic ablation or pharmacological blockade of C3/C3aR signaling pathway suppressed neuroinflammation and attenuated cognitive declines in PND. The C3/C3aR axis is essential for surgery-induced platelet count and circulating PF4 declines, and mice supplemented with recombinant PF4 exhibited reduced neuroinflammation and improved cognitive function. Together, our findings revealed the new roles of the C3/C3aR signaling pathway in platelet dysfunction and neuroinflammation in age-related PND, and these results highlight new potential therapeutic strategies for PND.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All materials and raw data are available from the corresponding authors upon reasonable request.
References
Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30.
Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150:1134–40.
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–12.
Berger M, Schenning KJ, Brown CHT, Deiner SG, Whittington RA, Eckenhoff RG, et al. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg. 2018;127:1406–13.
Deiner S, Liu X, Lin HM, Jacoby R, Kim J, Baxter MG, et al. Does postoperative cognitive decline result in new disability after surgery? Ann Surg. 2021;274:e1108–e1114.
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32.
Barreto Chang OL, Possin KL, Maze M. Age-related perioperative neurocognitive disorders: experimental models and druggable targets. Annu Rev Pharmacol Toxicol. 2023;63:321–40.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128:781–8.
Lu J, Hou W, Gao S, Zhang Y, Zong Y. The role of gut microbiota-gut-brain axis in perioperative neurocognitive dysfunction. Front Pharmacol. 2022;13:879745.
Rust C, Malan-Muller S, van den Heuvel LL, Tonge D, Seedat S, Pretorius E, et al. Platelets bridging the gap between gut dysbiosis and neuroinflammation in stress-linked disorders: a narrative review. J Neuroimmunol. 2023;382:578155.
Lai Z, Shan W, Li J, Min J, Zeng X, Zuo Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol Psychiatry. 2021;26:7167–87.
Luo A, Li S, Wang X, Xie Z, Li S, Hua D. Cefazolin improves anesthesia and surgery-induced cognitive impairments by modulating blood-brain barrier function, gut bacteria and short chain fatty acids. Front Aging Neurosci. 2021;13:748637.
VanDusen KW, Li YJ, Cai V, Hall A, Hiles S, Thompson JW, et al. Cerebrospinal fluid proteome changes in older non-cardiac surgical patients with postoperative cognitive dysfunction. J Alzheimers Dis. 2021;80:1281–97.
Xiong C, Liu J, Lin D, Zhang J, Terrando N, Wu A. Complement activation contributes to perioperative neurocognitive disorders in mice. J Neuroinflammation. 2018;15:254.
Leiter O, Seidemann S, Overall RW, Ramasz B, Rund N, Schallenberg S, et al. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Reports. 2019;12:667–79.
Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–7.
Amelot AA, Tagzirt M, Ducouret G, Kuen RL, Le Bonniec BF. Platelet factor 4 (CXCL4) seals blood clots by altering the structure of fibrin. J Biol Chem. 2007;282:710–20.
Nevzorova TA, Mordakhanova ER, Daminova AG, Ponomareva AA, Andrianova IA, Le Minh G, et al. Platelet factor 4-containing immune complexes induce platelet activation followed by calpain-dependent platelet death. Cell Death Discov. 2019;5:106.
Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res. 2010;125:292–6.
Park C, Hahn O, Gupta S, Moreno AJ, Marino F, Kedir B, et al. Platelet factors are induced by longevity factor klotho and enhance cognition in young and aging mice. Nat Aging. 2023;3:1067–78.
Schroer AB, Ventura PB, Sucharov J, Misra R, Chui MKK, Bieri G, et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature. 2023;620:1071–9.
Leiter O, Brici D, Fletcher SJ, Yong XLH, Widagdo J, Matigian N, et al. Platelet-derived exerkine CXCL4/platelet factor 4 rejuvenates hippocampal neurogenesis and restores cognitive function in aged mice. Nat Commun. 2023;14:4375.
Nizzardo M, Nardini M, Ronchi D, Salani S, Donadoni C, Fortunato F, et al. Beta-lactam antibiotic offers neuroprotection in a spinal muscular atrophy model by multiple mechanisms. Exp Neurol. 2011;229:214–25.
Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7.
Tikhonova MA, Amstislavskaya TG, Ho YJ, Akopyan AA, Tenditnik MV, Ovsyukova MV, et al. Neuroprotective effects of ceftriaxone involve the reduction of abeta burden and neuroinflammatory response in a mouse model of Alzheimer’s disease. Front Neurosci. 2021;15:736786.
Liang P, Shan W, Zuo Z. Perioperative use of cefazolin ameliorates postoperative cognitive dysfunction but induces gut inflammation in mice. J Neuroinflammation. 2018;15:235.
Carson N, Leach L, Murphy KJ. A re-examination of montreal cognitive assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33:379–88.
Liu Y, Fu H, Wang T. Neuroinflammation in perioperative neurocognitive disorders: from bench to the bedside. CNS Neurosci Ther. 2022;28:484–96.
Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J Neurosci. 2016;36:577–89.
Mou W, Ma L, Zhu A, Cui H, Huang Y. Astrocyte-microglia interaction through C3/C3aR pathway modulates neuropathic pain in rats model of chronic constriction injury. Mol Pain. 2022;18:17448069221140532.
Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198.
Fu H, Hardy J, Duff KE. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21:1350–8.
Macaluso A, Bernabucci M, Trabucco A, Ciolli L, Troisi F, Baldini R, et al. Analgesic effect of a single preoperative dose of the antibiotic ceftriaxone in humans. J Pain. 2013;14:604–12.
Jain H, Singh G, Gambhir HS. Cefazolin-associated hallucinations in a young male with normal renal function. Am J Ther. 2023;30:168–70.
Chen CC, Lee DJ, Lin SH. Reversible cefazolin-induced status epilepticus in a peritoneal dialysis patient. Toxicol Rep. 2022;9:1950–2.
Lavillaureix J, Brogard JM, Pinget M, Ledoux F. Dosage adjustments of cefazolin according to the pharmacokinetics of this new cephalosporin. Infection. 1975;3:105–14.
Scully BE, Fu KP, Neu HC. Pharmacokinetics of ceftriaxone after intravenous infusion and intramuscular injection. Am J Med. 1984;77:112–6.
Gao R, Ali T, Liu Z, Li A, Hao L, He L, et al. Ceftriaxone averts neuroinflammation and relieves depressive-like behaviors via GLT-1/TrkB signaling. Biochem Biophys Res Commun. 2024;701:149550.
Liu LZ, Fan SJ, Gao JX, Li WB, Xian XH. Ceftriaxone ameliorates hippocampal synapse loss by inhibiting microglial/macrophages activation in glial glutamate transporter-1 dependent manner in the APP/PS1 mouse model of Alzheimer’s disease. Brain Res Bull. 2023;200:110683.
Lim SW, Su HC, Nyam TE, Chio CC, Kuo JR, Wang CC. Ceftriaxone therapy attenuates brain trauma in rats by affecting glutamate transporters and neuroinflammation and not by its antibacterial effects. BMC Neurosci. 2021;22:54.
Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39.
Wu M, Zheng W, Song X, Bao B, Wang Y, Ramanan D, et al. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell. 2024;187:897–913.e818.
Ngo ATP, Skidmore A, Oberg J, Yarovoi I, Sarkar A, Levine N, et al: Platelet factor 4 limits neutrophil extracellular trap– and cell-free DNA–induced thrombogenicity and endothelial injury. JCI Insight 2023;8:e171054.
Podolnikova NP, Lishko VK, Roberson R, Koh Z, Derkach D, Richardson D, et al. Platelet factor 4 improves survival in a murine model of antibiotic-susceptible and methicillin-resistant staphylococcus aureus peritonitis. Front Cell Infect Microbiol. 2023;13:1217103.
Goff LM, Davies K, Zelek WM, Kodosaki E, Hakim O, Lockhart S, et al. Ethnic differences in complement system biomarkers and their association with metabolic health in men of Black African and White European ethnicity. Clin Exp Immunol. 2023;212:52–60.
Bao N, Liu J, Peng Z, Zhang R, Ni R, Li R, et al. Identification of circRNA-miRNA-mRNA networks to explore the molecular mechanism and immune regulation of postoperative neurocognitive disorder. Aging. 2022;14:8374–93.
Acknowledgements
We thank Dr. M. Gao for his technical assistance and for generous gift of CR2-crry. Z.L. received support from Youth Talents Project of the Fujian Young Eagle Program (grant No. FJHRSS2022173), Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare (grant No. FJHCHR202326), National Natural Science Foundation of China (grant No.82171201), Joint Funds for the Innovation of Science and Technology of Fujian Province, China (grant No. 2021Y9073), Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (grant No. 2023ZQNZD003), Z.Z. was supported by the Talent Program of Hubei University of Technology (grant No. GCC2024015). J.W. received support from the Startup Fund for Scientific Research, Fujian Medical University (Grant No. 2024QH1032).
Author information
Authors and Affiliations
Contributions
ZL, SW, and JW conceived and designed the study. JW, CY, SL, ML, XR, NZ, WW, ZT, MO, ZZ, ML, LZ, and SH performed the experiments. KZ, JW, JZ, JJZ, and CY conducted data analysis and visualization. PZ, HZ, ZL, and LP managed the project. ZL, SW, and JW drafted the original manuscript. ZZ contributed to the conceptualization and preparation of the revised manuscript. All authors reviewed and edited the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, JL., Ye, CH., Teng, ZF. et al. Targeting complement C3/C3aR pathway restores rejuvenation factor PF4 and mitigates neurocognitive impairments in age-related perioperative neurocognitive disorders. Mol Psychiatry 30, 5192–5202 (2025). https://doi.org/10.1038/s41380-025-03103-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03103-z