Abstract
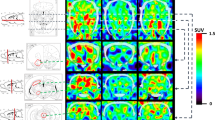
Histone deacetylases (HDACs), typically known for regulating gene expression, also play a major role in protein regulation outside of histone modification. Emerging evidence suggests the HDACs may be novel pharmacologic targets in complex disorders such as posttraumatic stress disorder (PTSD). Histone deacetylase 6 (HDAC6) regulates microtubule function and plays a role in stress-related cortisol signaling in serotonergic regions of the brain by maintaining the nuclear translocation of glucocorticoid receptors. Here, we report results of a translational positron emission tomography brain imaging study using a novel HDAC6-selective radiotracer, [18F]Bavarostat. In humans, we demonstrate significantly lower availability of HDAC6 in the amygdala of individuals with PTSD compared to non-trauma exposed controls. These proof-of-concept human findings are supported by rodent findings of reduced HDAC6 availability both in case-control groups and within-subject longitudinal analysis using a single prolonged stress model. Together, our translational findings demonstrate a potential role for HDAC6 in the pathophysiology of PTSD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Due to the sensitive nature of human participant information, data are available upon reasonable request by contacting the corresponding author.
Code availability
Computer codes are written and property of Yale PET Center and can be accessed upon request.
References
Kirkpatrick HA, Heller GM. Post-traumatic stress disorder: theory and treatment update. Int J Psychiatry Med. 2014;47:337–46.
Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med. 2017;47:2260–74.
Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057.
Al Jowf GI, Ahmed ZT, Reijnders RA, de Nijs L, Eijssen LMT. To predict, prevent, and manage Post-Traumatic Stress Disorder (PTSD): a review of pathophysiology, treatment, and biomarkers. Int J Mol Sci. 2023;24:5238.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60.
Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur J Psychotraumatol. 2017;8:1351204.
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8.
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–31.
Saunders HAJ, Johnson-Schlitz DM, Jenkins BV, Volkert PJ, Yang SZ, Wildonger J. Acetylated α-tubulin K394 regulates microtubule stability to shape the growth of axon terminals. Curr Biol. 2022;32:614–30.e615.
Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci. 2012;32:4400–16.
Jochems J, Teegarden SL, Chen Y, Boulden J, Challis C, Ben-Dor GA, et al. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol Psychiatry. 2015;77:345–55.
Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–7.
Winkler R, Benz V, Clemenz M, Bloch M, Foryst-Ludwig A, Wardat S, et al. Histone deacetylase 6 (HDAC6) is an essential modifier of glucocorticoid-induced hepatic gluconeogenesis. Diabetes. 2012;61:513–23.
Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39:389–400.
Xie S-T, Chen A-X, Song B, Fan J, Li W, Xing Z, et al. Suppression of microglial activation and monocyte infiltration ameliorates cerebellar hemorrhage induced-brain injury and ataxia. Brain Behavior Immun. 2020;89:400–13.
Yan B, Xie S, Liu Y, Liu W, Li D, Liu M, et al. Histone deacetylase 6 modulates macrophage infiltration during inflammation. Theranostics. 2018;8:2927–38.
Youn GS, Park JK, Lee CY, Jang JH, Yun SH, Kwon HY, et al. MicroRNA-22 negatively regulates LPS-induced inflammatory responses by targeting HDAC6 in macrophages. BMB Rep. 2020;53:223–8.
Liu JR, Yu CW, Hung PY, Hsin LW, Chern JW. High-selective HDAC6 inhibitor promotes HDAC6 degradation following autophagy modulation and enhanced antitumor immunity in glioblastoma. Biochem Pharmacol. 2019;163:458–71.
Celen S, Rokka J, Gilbert TM, Koole M, Vermeulen I, Serdons K, et al. Translation of HDAC6 PET imaging using [(18)F]EKZ-001-cGMP production and measurement of HDAC6 target occupancy in nonhuman primates. ACS Chem Neurosci. 2020;11:1093–101.
Koole M, Van Weehaeghe D, Serdons K, Herbots M, Cawthorne C, Celen S, et al. Clinical validation of the novel HDAC6 radiotracer [(18)F]EKZ-001 in the human brain. Eur J Nucl Med Mol Imaging. 2020;48:596–611.
Strebl MG, Campbell AJ, Zhao W-N, Schroeder FA, Riley MM, Chindavong PS, et al. HDAC6 brain mapping with [18F]Bavarostat enabled by a ru-mediated deoxyfluorination. ACS Cent Sci. 2017;3:1006–14.
Seibenhener ML, Wooten MC Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 2015:e52434. https://doi.org/10.3791/52434.
De Carvalho LM, Wiers CE, Sun H, Wang GJ, Volkow ND. Increased transcription of TSPO, HDAC2, and HDAC6 in the amygdala of males with alcohol use disorder. Brain Behav. 2020;11:e01961.
Hannestad J, DellaGioia N, Gallezot J-D, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C] PBR28 PET study. Brain Behav Immun. 2013;33:131–8.
Ceccarini G, Flavell RR, Butelman ER, Synan M, Willnow TE, Bar-Dagan M, et al. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10:148–59.
First MB, Gibbon M The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Hilsenroth M J, D L Segal editors. Comprehensive handbook of psychological assessment Personality assessment. John Wiley & Sons, Inc. 2004. Vol. 2. pp. 134–143.
First MB. Structured clinical interview for the DSM (SCID). Encycl Clin Psychol. In: R L Cautin and S O Lilienfeld editors. The Encyclopedia of Clinical Psychology 2015;1-6 https://doi.org/10.1002/9781118625392.wbecp351.
Sobell LC, Sobell MB Timeline follow-back. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press, 1992, pp 41-72.
Naganawa M, Zheng MQ, Gallezot JD, Bonomi R, Gu J, Gao H, et al. Assessment of test-retest reproducibility by [18F]Bavarostat for PET imaging of HDAC6. EJNMMI Res. 2025;15:76.
Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605.
Jin X, Chan C, Mulnix T, Panin V, Casey ME, Liu C, et al. List-mode reconstruction for the Biograph mCT with physics modeling and event-by-event motion correction. Phys Med Biol. 2013;58:5567–91.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480:16–20.
Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA. Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front Psychiatry. 2018;9:196.
Gould TD, Dao DT, Kovacsics CE The open field test. Mood and anxiety related phenotypes in mice: characterization using behavioral tests 2009; 1-20.
Barrière DA, Magalhães R, Novais A, Marques P, Selingue E, Geffroy F, et al. The SIGMA rat brain templates and atlases for multimodal MRI data analysis and visualization. Nat Commun. 2019;10:5699.
Pratchett LC, Yehuda R. Foundations of posttraumatic stress disorder: does early life trauma lead to adult posttraumatic stress disorder? Dev Psychopathol. 2011;23:477–91.
Eagle AL, Fitzpatrick CJ, Perrine SA. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res. 2013;256:591–7. https://doi.org/10.1016/j.bbr.2013.1009.1014
Zhang L, Hu XZ, Li H, Li X, Yu T, Dohl J, et al. Updates in PTSD animal models characterization. Methods Mol Biol. 2019;2011:331–44.
Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, et al. Loss of deacetylation activity of Hdac6 affects emotional behavior in mice. PLoS One. 2012;7:e30924.
Iaconelli J, Xuan L, Karmacharya R. HDAC6 modulates signaling pathways relevant to synaptic biology and neuronal differentiation in human stem-cell-derived neurons. Int J Mol Sci. 2019;20:1605.
Singh H, Wray N, Schappi JM, Rasenick MM. Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds. Neuropsychopharmacology. 2018;43:1481–91.
Li Y, Zhang B, Yang Y, Su P, Samsom JN, Wong AHC, et al. Sex and age differences in glucocorticoid signaling after an aversive experience in mice. Cells. 2024;13:2041.
Li H, Su P, Lai TK, Jiang A, Liu J, Zhai D, et al. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J Clin Invest. 2020;130:877–89.
Bhatt S, Hillmer AT, Rusowicz A, Nabulsi N, Matuskey D, Angarita GA, et al. Imaging brain cortisol regulation in PTSD with a target for 11β-hydroxysteroid dehydrogenase type 1. J Clin Invest. 2021;131:e150452.
Keller SM, Schreiber WB, Staib JM, Knox D. Sex differences in the single prolonged stress model. Behav Brain Res. 2015;286:29–32.
Bielajew C, Konkle AT, Merali Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res. 2002;136:583–92.
Acknowledgements
We are grateful for the expertise of the staff at the Yale PET Center and Yale PET animal facility, for their support of radiochemistry and imaging, particularly Dr. Takuya Toyonaga and Krista Fowles. We thank Alexa-Nicole Leverington, Rosemarie Terwilliger, and the animal staff at the Yale Animal Facilities and Charles Rivers. Additionally, we thank the Veterans Affairs National Center for PTSD for significant support of this work. We are thankful for our funding from the National Institute of Mental Health grants 5T32MH019961-24, the Veterans Affairs National Center for PTSD (KPC, MJG), and the Thomas P. Detre Award for Neurosciences (REB). This work was supported with resources and use of facilities at the VA Connecticut Health Care System, West Haven, CT and the National Center for PTSD, U.S. Department of Veterans Affairs. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs (VA) or the U.S. government or the views of the Department of Mental Health and Addiction Services or the State of Connecticut.
Author information
Authors and Affiliations
Contributions
REB, KPC, and MJG conceived and designed the study. REB and DM conducted human subjects’ recruitment, medical clearance, characterization, and oversight. REB conducted all animal studies and behavior analysis. REB and MN analyzed the PET data with input from REC and RP REB performed animal studies and CD performed immunohistochemistry with oversight from MJG REB analyzed rodent and human PET, and rodent behavior. REB, MJG, RP, and KPC wrote the manuscript. All authors edited and contributed comments and intellectual content to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bonomi, R.E., Naganawa, M., McRiley, D. et al. PET imaging evidence of HDAC6 suppression in the amygdala across species in PTSD. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03124-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03124-8