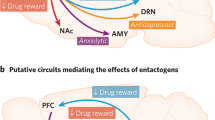
Abstract
Psychedelics, particularly psilocybin, have garnered significant attention as potential therapeutic tools for treating substance use disorders (SUDs), such as those related to alcohol, nicotine, heroin (an opioid), or cocaine. Traditional treatments often fall short, leading to high relapse rates and an urgent need for innovative approaches. This article explores the emerging role of psychedelics in SUDs therapy, highlighting their ability to disrupt maladaptive neural circuits, promote neuroplasticity, and facilitate profound psychological insights that address the root causes of SUDs. Clinical trials demonstrate promising results across various forms of SUDs, with psilocybin-assisted therapy showing significant reductions in substance use and improved mental health outcomes. Despite the potential, challenges such as legal barriers, safety concerns, and the need for more rigorous research remain. The future of psychedelics in SUDs treatment is cautiously optimistic, with the possibility of transforming the field of SUDs therapy and offering hope to millions of individuals struggling with SUDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry. 2013;170:834–51.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86.
Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug. Alcohol. Depend. 2021;218:108350.
Volkow ND, Blanco C. Medications for opioid use disorders: clinical and pharmacological considerations. J. Clin. Invest. 2020;130:10–13.
McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95.
Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology. 2017;42:2105–13.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
van der Meer PB, Fuentes JJ, Kaptein AA, Schoones JW, de Waal MM, Goudriaan AE, et al. Therapeutic effect of psilocybin in addiction: A systematic review. Front. Psychiatry. 2023;14:1134454.
Dyck E. Hitting highs at rock bottom’: LSD treatment for alcoholism, 1950–1970. Soc. Hist. Med. 2006;19:313–29.
Kaplan RM. Humphry Fortescue Osmond (1917-2004), a radical and conventional psychiatrist: The transcendent years. J. Med. Biogr. 2016;24:115–24.
Vollenweider FX, Preller KH. Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 2020;21:611–24.
Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl Acad. Sci. USA. 2016;113:4853–8.
Olson DE. Toward translatable biomarkers of psychedelic-induced neuroplasticity. Am. J. Psychiatry. 2025;182:10–12.
Vamvakopoulou IA, Nutt DJ. Psychedelics: from cave art to 21st-century medicine for addiction. Eur. Addict. Res. 2024;30:302–20.
Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, et al. Structure of a hallucinogen-activated Gq-coupled 5-HT(2A) serotonin receptor. Cell. 2020;182:1574–88 e1519.
Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. 2023;379:700–6.
McClure-Begley TD, Roth BL. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug. Discov. 2022;21:463–73.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337–8.
Slomski A. Ketamine to help treat cocaine use disorder. JAMA. 2019;322:717.
Glick SD, Maisonneuve IM, Pearl SM. Evidence for roles of kappa-opioid and NMDA receptors in the mechanism of action of ibogaine. Brain Res. 1997;749:340–3.
Coleman JA, Yang D, Zhao Z, Wen PC, Yoshioka C, Tajkhorshid E, et al. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature. 2019;569:141–5.
Grieco SF, Castren E, Knudsen GM, Kwan AC, Olson DE, Zuo Y, et al. Psychedelics and neural plasticity: therapeutic implications. J. Neurosci. 2022;42:8439–49.
Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009;2009:CD002209.
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry. 2008;165:179–87.
Schindler EAD, D’Souza DC. The therapeutic potential of psychedelics. Science. 2022;378:1051–3.
Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 2015;29:289–99.
Bogenschutz MP, Ross S, Bhatt S, Baron T, Forcehimes AA, Laska E, et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79:953–62.
Tap SC. The potential of 5-methoxy-N,N-dimethyltryptamine in the treatment of alcohol use disorder: a first look at therapeutic mechanisms of action. Addict. Biol. 2024;29:e13386.
Floris G, Dabrowski KR, Zanda MT, Daws SE. Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats. Mol. Psychiatry. 2024;30:1801–16.
Nichols DE, Johnson MW, Nichols CD. Psychedelics as medicines: an emerging new paradigm. Clin. Pharmacol. Ther. 2017;101:209–19.
Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol. Rev. 2019;71:316–44.
Pagni BA, Petridis PD, Podrebarac SK, Grinband J, Claus ED, Bogenschutz MP. Psilocybin-induced changes in neural reactivity to alcohol and emotional cues in patients with alcohol use disorder: an fMRI pilot study. Sci. Rep. 2024;14:3159.
Reinwald JR, Schmitz CN, Skorodumov I, Kuchar M, Weber-Fahr W, Spanagel R, et al. Psilocybin-induced default mode network hypoconnectivity is blunted in alcohol-dependent rats. Transl. Psychiatry. 2023;13:392.
Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug. Alcohol. Abuse. 2017;43:55–60.
Elsila LV, Harkki J, Enberg E, Martti A, Linden AM, Korpi ER. Effects of acute lysergic acid diethylamide on intermittent ethanol and sucrose drinking and intracranial self-stimulation in C57BL/6 mice. J. Psychopharmacol. 2022;36:860–74.
Alper K, Dong B, Shah R, Sershen H, Vinod KY. LSD administered as a single dose reduces alcohol consumption in C57BL/6J mice. Front. Pharmacol. 2018;9:994.
De Gregorio D, Posa L, Ochoa-Sanchez R, McLaughlin R, Maione S, Comai S, et al. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT(1A), D(2) and TAAR(1) receptors. Pharmacol. Res. 2016;113:81–91.
Belgers M, Leenaars M, Homberg JR, Ritskes-Hoitinga M, Schellekens AF, Hooijmans CR. Ibogaine and addiction in the animal model, a systematic review and meta-analysis. Transl. Psychiatry. 2016;6:e826.
Arenson A, Campbell CI, Remler I. Psychoactive plant derivatives (ayahuasca, ibogaine, kratom) and their application in opioid withdrawal and use disorder - a narrative review. J. Addict. Dis. 2024;42:253–63.
Torrado Pacheco A, Olson RJ, Garza G, Moghaddam B. Acute psilocybin enhances cognitive flexibility in rats. Neuropsychopharmacology. 2023;48:1011–20.
Carhart-Harris RL, Nutt DJ. Serotonin and brain function: a tale of two receptors. J. Psychopharmacol. 2017;31:1091–120.
Benvenuti F, Colombo D, Soverchia L, Cannella N, Domi E, Ciccocioppo R. Psilocybin prevents reinstatement of alcohol seeking by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology. 2023;240:1521–30.
Shao LX, Liao C, Davoudian PA, Savalia NK, Jiang Q, Wojtasiewicz C, et al. Psilocybin’s lasting action requires pyramidal cell types and 5-HT(2A) receptors. Nature. 2025;642:411–20. https://doi.org/10.1038/s41586-025-08813-6
De Gregorio D, Popic J, Enns JP, Inserra A, Skalecka A, Markopoulos A, et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc. Natl Acad. Sci. USA. 2021;118:e2020705118.
Wallach J, Cao AB, Calkins MM, Heim AJ, Lanham JK, Bonniwell EM, et al. Identification of 5-HT(2A) receptor signaling pathways associated with psychedelic potential. Nat. Commun. 2023;14:8221.
Holz A, Mulsch F, Schwarz MK, Hollmann M, Dobrossy MD, Coenen VA, et al. Enhanced mglu5 signaling in excitatory neurons promotes rapid antidepressant effects via ampa receptor activation. Neuron. 2019;104:338–52 e337.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Moya-Alvarado G, Tiburcio-Felix R, Ibanez MR, Aguirre-Soto AA, Guerra MV, Wu C, et al. BDNF/TrkB signaling endosomes in axons coordinate CREB/mTOR activation and protein synthesis in the cell body to induce dendritic growth in cortical neurons. Elife. 2023;12:e77455.
Worrell SD, Gould TJ. Therapeutic potential of ketamine for alcohol use disorder. Neurosci. Biobehav. Rev. 2021;126:573–89.
Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol. Rev. 2021;73:202–77.
Sweetnam PM, Lancaster J, Snowman A, Collins JL, Perschke S, Bauer C, et al. Receptor binding profile suggests multiple mechanisms of action are responsible for ibogaine’s putative anti-addictive activity. Psychopharmacology. 1995;118:369–76.
He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20:2420–2.
Marton S, Gonzalez B, Rodriguez-Bottero S, Miquel E, Martinez-Palma L, Pazos M, et al. Ibogaine administration modifies gdnf and bdnf expression in brain regions involved in mesocorticolimbic and nigral dopaminergic circuits. Front. Pharmacol. 2019;10:193.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–44 e2534.
Strickland JC, Garcia-Romeu A, Johnson MW. Set and Setting: a randomized study of different musical genres in supporting psychedelic therapy. ACS Pharmacol. Transl. Sci. 2021;4:472–8.
Hartogsohn I. Set and setting, psychedelics and the placebo response: an extra-pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016;30:1259–67.
Heinzerling KG, Sergi K, Linton M, Rich R, Youssef B, Bentancourt I, et al. Nature-themed video intervention may improve cardiovascular safety of psilocybin-assisted therapy for alcohol use disorder. Front. Psychiatry. 2023;14:1215972.
MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J. Psychopharmacol. 2011;25:1453–61.
Calleja-Conde J, Morales-Garcia JA, Echeverry-Alzate V, Buhler KM, Gine E, Lopez-Moreno JA. Classic psychedelics and alcohol use disorders: a systematic review of human and animal studies. Addict. Biol. 2022;27:e13229.
Vanderijst L, Hever F, Buot A, Daure C, Benoit J, Hanak C, et al. Psilocybin-assisted therapy for severe alcohol use disorder: protocol for a double-blind, randomized, placebo-controlled, 7-month parallel-group phase II superiority trial. BMC Psychiatry. 2024;24:77.
Jensen ME, Stenbaek DS, Juul TS, Fisher PM, Ekstrom CT, Knudsen GM, et al. Psilocybin-assisted therapy for reducing alcohol intake in patients with alcohol use disorder: protocol for a randomised, double-blinded, placebo-controlled 12-week clinical trial (The QUANTUM Trip Trial). BMJ Open. 2022;12:e066019.
Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014;28:983–92.
Elsilä LV, Harkki J, Enberg E, Martti A, Linden AM, Korpi ER. Effects of acute lysergic acid diethylamide on intermittent ethanol and sucrose drinking and intracranial self-stimulation in C57BL/6 mice. J. Psychopharmacol. 2022;36:860–74.
Meinhardt MW, Gungor C, Skorodumov I, Mertens LJ, Spanagel R. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology. 2020;45:1316–22.
Fuentes JJ, Fonseca F, Elices M, Farre M, Torrens M. Therapeutic use of lsd in psychiatry: a systematic review of randomized-controlled clinical trials. Front. Psychiatry. 2019;10:943.
Knuijver T, ter Heine R, Schellekens AFA, Heydari P, Lucas L, Westra S, et al. The pharmacokinetics and pharmacodynamics of ibogaine in opioid use disorder patients. J. Psychopharmacol. 2024;38:481–8.
O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J. Neurosci. 1997;17:8828–41.
Koenig X, Hilber K. The anti-addiction drug ibogaine and the heart: a delicate relation. Molecules. 2015;20:2208–28.
Brown TK, Alper K. Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes. Am. J. Drug. Alcohol. Abuse. 2018;44:24–36.
Davis AK, Barsuglia JP, Windham-Herman AM, Lynch M, Polanco M. Subjective effectiveness of ibogaine treatment for problematic opioid consumption: short- and long-term outcomes and current psychological functioning. J. Psychedelic Stud. 2017;1:65–73.
He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci. 2005;25:619–28.
Vastag B. Ibogaine therapy: a ‘Vast, Uncontrolled Experiment’. Science. 2005;308:345–6.
Schenberg EE, de Castro Comis MA, Chaves BR, da Silveira DX. Treating drug dependence with the aid of ibogaine: a retrospective study. J. Psychopharmacol. 2014;28:993–1000.
Alper KR, Stajic M, Gill JR. Fatalities temporally associated with the ingestion of ibogaine. J. Forensic Sci. 2012;57:398–412.
Schep LJ, Slaughter RJ, Galea S, Newcombe D. Ibogaine for treating drug dependence. What is a safe dose? Drug. Alcohol. Depend. 2016;166:1–5.
Roberts E Hype or hope? The developing evidence base for psychedelic treatment of addiction disorders. Br J Psychiatry. 2025;1-3. https://doi.org/10.1192/bjp.2025.19
Koenig X, Kovar M, Boehm S, Sandtner W, Hilber K. Anti‐addiction drug ibogaine inhibits hERG channels: a cardiac arrhythmia risk. Addict. Biol. 2012;19:237–9.
Brett J, Knock E, Korthuis PT, Liknaitzky P, Murnane KS, Nicholas CR, et al. Exploring psilocybin-assisted psychotherapy in the treatment of methamphetamine use disorder. Front. Psychiatry. 2023;14:1123424.
Barber M, Gardner J, Savic M, Carter A. Ibogaine therapy for addiction: consumer views from online fora. Int. J. Drug. Policy. 2020;83:102857.
Nicholas CR, Wang JB, Coker A, Mitchell JM, Klaire SS, Yazar-Klosinski B, et al. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug. Alcohol. Depend. 2022;233:109356.
Rush B, Marcus O, Garcia S, Loizaga-Velder A, Loewinger G, Spitalier A, et al. Protocol for outcome evaluation of ayahuasca-assisted addiction treatment: the case of takiwasi center. Front. Pharmacol. 2021;12:659644.
Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474–9.
Bogenschutz MP, Podrebarac SK, Duane JH, Amegadzie SS, Malone TC, Owens LT, et al. Clinical interpretations of patient experience in a trial of psilocybin-assisted psychotherapy for alcohol use disorder. Front. Pharmacol. 2018;9:100.
Garcia-Romeu A, Davis AK, Erowid F, Erowid E, Griffiths RR, Johnson MW. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J. Psychopharmacol. 2019;33:1088–101.
Doering-Silveira E, Grob CS, de Rios MD, Lopez E, Alonso LK, Tacla C, et al. Report on psychoactive drug use among adolescents using ayahuasca within a religious context. J. Psychoact. Drugs. 2005;37:141–4.
Barsuglia JP, Polanco M, Palmer R, Malcolm BJ, Kelmendi B, Calvey T. A case report SPECT study and theoretical rationale for the sequential administration of ibogaine and 5-MeO-DMT in the treatment of alcohol use disorder. Prog. Brain Res. 2018;242:121–58.
Savage C, McCabe OL. Residential psychedelic (LSD) therapy for the narcotic addict. A controlled study. Arch. Gen. Psychiatry. 1973;28:808–14.
Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict. 1999;8:234–42.
Malcolm BJ, Polanco M, Barsuglia JP. Changes in withdrawal and craving scores in participants undergoing opioid detoxification utilizing ibogaine. J. Psychoact. Drugs. 2018;50:256–65.
Noller GE, Frampton CM, Yazar-Klosinski B. Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. Am. J. Drug. Alcohol. Abuse. 2018;44:37–46.
Mash DC, Duque L, Page B, Allen-Ferdinand K. Ibogaine detoxification transitions opioid and cocaine abusers between dependence and abstinence: clinical observations and treatment outcomes. Front. Pharmacol. 2018;9:529.
Knuijver T, Schellekens A, Belgers M, Donders R, van Oosteren T, Kramers K, et al. Safety of ibogaine administration in detoxification of opioid-dependent individuals: a descriptive open-label observational study. Addiction. 2021;117:118–28.
Wilkins C, dos Santos RG, Solá J, Aixalá M, Cura P, Moreno E, et al. Detoxification from methadone using low, repeated, and increasing doses of ibogaine: a case report. J. Psychedelic Stud. 2017;1:29–34.
Glue P, Cape G, Tunnicliff D, Lockhart M, Lam F, Hung N, et al. Ascending single-dose, double-blind, placebo-controlled safety study of noribogaine in opioid-dependent patients. Clin. Pharmacol. Drug. Dev. 2016;5:460–8.
Pisano VD, Putnam NP, Kramer HM, Franciotti KJ, Halpern JH, Holden SC. The association of psychedelic use and opioid use disorders among illicit users in the United States. J. Psychopharmacol. 2017;31:606–13.
Argento E, Socias ME, Hayashi K, Choi J, Mackay L, Christie D, et al. Psychedelic use is associated with reduced daily opioid use among people who use illicit drugs in a Canadian setting. Int. J. Drug. Policy. 2022;100:103518.
Jones GM, Nock MK. Exploring protective associations between the use of classic psychedelics and cocaine use disorder: a population-based survey study. Sci. Rep. 2022;12:2574.
Fábregas JM, González D, Fondevila S, Cutchet M, Fernández X, Barbosa PC, et al. Assessment of addiction severity among ritual users of ayahuasca. Drug. Alcohol. Depend. 2010;111:257–61.
Thomas G, Lucas P, Capler NR, Tupper KW, Martin G. Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr. Drug. Abuse Rev. 2013;6:30–42.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22207103, T2350008, T2341003), STI2030-Major Projects [2021ZD0203000 (2021ZD0203003)], International Partnership Program of the Chinese Academy of Sciences (029GJHZ2024057GC), and the Open Research Fund of the State Key Laboratory of Brain-Machine Intelligence, Zhejiang University (Grant No. BMI2400014).
Author information
Authors and Affiliations
Contributions
YL and HL: organizational framework and construction, paper drafting; HW: paper drafting and revision; XW: proposal, design and final revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Li, H., Wang, H. et al. Exploring the therapeutic potential of psychedelics in treating substance use disorders. Mol Psychiatry 30, 6134–6143 (2025). https://doi.org/10.1038/s41380-025-03168-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03168-w