Abstract
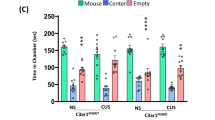
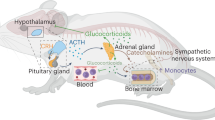
Post-traumatic stress disorder (PTSD) occurs in individuals who have experienced a traumatic event; however, not everyone who has experienced a traumatic event will develop this condition, highlighting the significance of susceptibility factors. Social hierarchy is a critical behavioral regulator of stress susceptibility and a risk factor for mental disorders. Individual resilience, the ability to recover from acute stress and preclinical injury, also plays a role in PTSD susceptibility. Some studies found that individuals with higher social rank were more resilient. Furthermore, individuals of a lower social rank exhibit increased microglial activity and the release of pro-inflammatory mediators, such as tumor necrosis factor-α (TNF-α); however, their exact role in PTSD remains unclear. Research has indicated that the nuclear factor kappa B (NF-κB) signaling pathway in the medial prefrontal cortex (mPFC) was activated abnormally in PTSD and correlated with changes in ubiquitin-like modifier activating enzyme 7 (Uba7) gene expression. However, the specific roles of Uba7 and the NF-κB pathway in PTSD susceptibility necessitate further investigation. Understanding these factors is crucial for comprehending the psychopathological changes in PTSD and developing preventive strategies. Our study validated three PTSD phenotypes using the mouse single prolonged stress (SPS) model, finding that resilience influenced PTSD recovery over time. Subsequently, we employed the chronic social defeat stress (CSDS) model to evaluate differential stress responses and recovery patterns over 14 days in mice of various social hierarchies and examined the related molecular changes in their mPFC. The results indicated that social hierarchy predicted PTSD susceptibility, with dominant individuals exhibiting greater vulnerability and more severe initial symptoms. Over time, resilient individuals in the lower-ranked groups recovered faster from anxiety and depression than those in the higher-ranked groups. Overactivation of microglia in the mPFC of susceptible individuals was associated with increased expression of NF-κB p65 and signals transducer and activator of transcription (STAT1) proteins, Uba7 gene expression, and TNF-α. Our findings highlight the complex interplay between social status and PTSD risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions of the present study are present in the main paper and/or the Supplementary Materials. Additional data are available from the corresponding author upon reasonable request.
References
Ben Barnes J, Presseau C, Jordan AH, Kline NK, Young-McCaughan S, Keane TM, et al. Common data elements in the assessment of military-related PTSD research applied in the consortium to alleviate PTSD. Mil Med. 2019;184:e218–e226.
Fitzgerald JM, DiGangi JA, Phan KL. Functional neuroanatomy of emotion and its regulation in PTSD. Harv Rev Psychiatry. 2018;26:116–28.
Wolkenstein L, Sommerhoff A, Voss M. Positive emotion dysregulation in posttraumatic stress disorder. J Anxiety Disord. 2022;86:102534.
Rakesh G, Morey RA, Zannas AS, Malik Z, Marx CE, Clausen AN, et al. Resilience as a translational endpoint in the treatment of PTSD. Mol Psychiatry. 2019;24:1268–83.
Osório C, Probert T, Jones E, Young AH, Robbins I. Adapting to stress: understanding the neurobiology of resilience. Behav Med. 2017;43:307–22.
Li H-Y, Zhu M-Z, Yuan X-R, Guo Z-X, Pan Y-D, Li Y-Q, et al. A thalamic-primary auditory cortex circuit mediates resilience to stress. Cell. 2023;186:1352–1368. e1318.
Willmore L, Cameron C, Yang J, Witten IB, Falkner AL. Behavioural and dopaminergic signatures of resilience. Nature. 2022;611:124–32.
Shanazz K, Nalloor R, Lucas R, Vazdarjanova A. Neuroinflammation is a susceptibility factor in developing a PTSD-like phenotype. Front Behav Neurosci. 2023;17:1112837.
Alexander KS, Nalloor R, Bunting KM, Vazdarjanova A. Investigating individual pre-trauma susceptibility to a PTSD-like phenotype in animals. Front Syst Neurosci. 2020;13:85.
Ma M, Xiong W, Hu F, Deng M-F, Huang X, Chen J-G, et al. A novel pathway regulates social hierarchy via lncRNA AtLAS and postsynaptic synapsin IIb. Cell Res. 2020;30:105–18.
LeClair KB, Chan KL, Kaster MP, Parise LF, Burnett CJ, Russo SJ. Individual history of winning and hierarchy landscape influence stress susceptibility in mice. Elife. 2021;10:e71401.
Šabanović M, Liu H, Mlambo V, Aqel H, Chaudhury D. What it takes to be at the top: the interrelationship between chronic social stress and social dominance. Brain Behav. 2020;10:e01896.
Liao J, Zeng G, Fang W, Huang W, Dai X, Ye Q, et al. Increased Notch2/NF-κB signaling may mediate the depression susceptibility: Evidence from chronic social defeat stress mice and WKY rats. Physiol Behav. 2021;228:113197.
Bollinger JL, Bergeon Burns CM, Wellman CL. Chronic restraint stress induces microglial activation, reflected in altered immune factor expression, in mPFC in male rats. Brain Behav Immun. 2016;52:88–97.
Bollinger JL, Burns CMB, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97.
Lguensat A, Bentefour Y, Bennis M, Ba-M’Hamed S, Garcia R. Susceptibility and resilience to PTSD-like symptoms in mice are associated with opposite dendritic changes in the prelimbic and infralimbic cortices following trauma. Neuroscience. 2019;418:166–76.
Cotrone TS, Hocog CB, Ramsey JT, Sanchez MA, Sullivan HM, Scrimgeour AG. Phenotypic characterization of frontal cortex microglia in a rat model of post‐traumatic stress disorder. Brain Behav. 2021;11:e02011.
Yang S, Xu K, Xu X, Zhu J, Jin Y, Liu Q, et al. S-Ketamine pretreatment alleviates anxiety-like behaviors and mechanical allodynia and blocks the pro-inflammatory response in striatum and periaqueductal gray from a post-traumatic stress disorder model. Front Behav Neurosci. 2022;16:848232.
Wang C, Fan L, Khawaja RR, Liu B, Zhan L, Kodama L, et al. Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat Commun. 2022;13:1969.
Girgenti MJ, Wang J, Ji D, Cruz DA, Stein MB, Gelernter J, et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci. 2021;24:24–33.
Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B, et al. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med. 2014;92:239–54.
Przanowski P, Loska S, Cysewski D, Dabrowski M, Kaminska B. ISG’ylation increases stability of numerous proteins including Stat1, which prevents premature termination of immune response in LPS-stimulated microglia. Neurochem Int. 2018;112:227–33.
Gupta S, Guleria RS. Involvement of nuclear factor-κB in inflammation and neuronal plasticity associated with post-traumatic stress disorder. Cells. 2022;11:2034.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
George SA, Stout SA, Tan M, Knox D, Liberzon I. Early handling attenuates enhancement of glucocorticoid receptors in the prefrontal cortex in an animal model of post-traumatic stress disorder. Biol Mood Anxiety Disord. 2013;3:22.
Wang F, Zhu J, Zhu H, Zhang Q, Hu H, Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–7.
Fulenwider HD, Caruso MA, Ryabinin AE. Manifestations of domination: assessments of social dominance in rodents. Genes Brain Behav. 2022;21:e12731.
Bhatnagar S. Rethinking stress resilience. Trends Neurosci. 2021;44:936–45.
Jiang Y, Wang Y, Sun X, Lian B, Sun H, Wang G, et al. Short- and long-term antidepressant effects of ketamine in a rat chronic unpredictable stress model. Brain Behav. 2017;7:e00749.
Li S, Liao Y, Dong Y, Li X, Li J, Cheng Y, et al. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J Neuroinflammation. 2021;18:1–14.
Malikowska-Racia N, Sałat K, Nowaczyk A, Fijałkowski Ł, Popik P. Dopamine D2/D3 receptor agonists attenuate PTSD-like symptoms in mice exposed to single prolonged stress. Neuropharmacology. 2019;155:1–9.
Jacobson ML, Wulf HA, Browne CA, Lucki I. The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology. 2020;237:3715–28.
Li Y, Du Y, Wang C, Lu G, Sun H, Kong Y, et al. (2R, 6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models. Neurobiol Stress. 2022;21:100503.
Zhang X, Zhao Y, Du Y, Sun H, Zhang W, Wang A, et al. Effect of ketamine on mood dysfunction and spatial cognition deficits in PTSD mouse models via HCN1–BDNF signaling. J Affect Disorders. 2021;286:248–58.
Sun H, Zhang X, Kong Y, Gou L, Lian B, Wang Y, et al. Maternal separation-induced histone acetylation correlates with BDNF-Programmed synaptic changes in an animal model of PTSD with sex differences. Mol Neurobiol. 2021;58:1738–54.
Xi K, Xiao H, Huang X, Yuan Z, Liu M, Mao H, et al. Reversal of hyperactive higher-order thalamus attenuates defensiveness in a mouse model of PTSD. Sci Adv. 2023;9:eade5987.
Holt LM, Gyles TM, Parise EM, Minier-Toribio AM, Rivera M, Markovic T, et al. Astrocytic CREB in Nucleus Accumbens Promotes Susceptibility to Chronic Stress. Biol Psychiatry. 2025;97:862–73.
Li L, Durand-de Cuttoli R, Aubry AV, Burnett CJ, Cathomas F, Parise LF, et al. Social trauma engages lateral septum circuitry to occlude social reward. Nature. 2023;613:696–703.
Litz BT. Resilience in the aftermath of war trauma: a critical review and commentary. Interface Focus. 2014;4:20140008.
Fan Z, Chang J, Liang Y, Zhu H, Zhang C, Zheng D, et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell. 2023;186:560–576. e517.
Larrieu T, Sandi C. Stress‐induced depression: is social rank a predictive risk factor? BioEssays. 2018;40:1800012.
LeClair KB, Russo SJ. Using social rank as the lens to focus on the neural circuitry driving stress coping styles. Curr Opin Neurobiol. 2021;68:167–80.
Park J-S, Heo H, Kim M-S, Lee S-E, Park S, Kim K-H, et al. Amphiregulin normalizes altered circuit connectivity for social dominance of the CRTC3 knockout mouse. Mol Psychiatry. 2023;28:4655–65.
Wood SK, Bhatnagar S. Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164–73.
Ishikawa R, Uchida C, Kitaoka S, Furuyashiki T, Kida S. Improvement of PTSD-like behavior by the forgetting effect of hippocampal neurogenesis enhancer memantine in a social defeat stress paradigm. Molecular Brain. 2019;12:1–6.
Yin Y-Y, Lai Z-K, Yan J-Z, Wei Q-Q, Wang B, Zhang L-M, et al. The interaction between social hierarchy and depression/anxiety: Involvement of glutamatergic pyramidal neurons in the medial prefrontal cortex (mPFC). Neurobiol Stress. 2023;24:100536.
Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–82.
Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KM, De Almeida RM. Microglial over-activation by social defeat stress contributes to anxiety-and depressive-like behaviors. Front Behav Neurosci. 2017;11:207.
Lier J, Streit WJ, Bechmann I. Beyond activation: characterizing microglial functional phenotypes. Cells. 2021;10:2236.
Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-α secretion. Brain Behav Immun. 2005;19:311–7.
Przanowski P, Loska S, Cysewski D, Dabrowski M, Kaminska B. ISG’ylation increases stability of numerous proteins including Stat1,which prevents premature termination of immune response in LPSstimulated microglia. Neurochem Int. 2018;112:227–33.
Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. Neural mechanisms linking social status and inflammatory responses to social stress. Soc Cogn Affec Neurosci. 2016;11:915–22.
Barnum CJ, Blandino P Jr, Deak T. Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain Behav Immun. 2008;22:517–27.
Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) 82101588, the Education and Teaching Reform Project of the Psychology and Education Reference Committee of the Ministry of Education 20221013, the Surface project of the Natural Science Foundation of Shandong Province ZR2020MC218, the Institute of Psychology, Chinese Academy of Sciences GJ202002, and the Graduate Student Research Grant from Weifang Medical University.
Author information
Authors and Affiliations
Contributions
L.P.G. and L.S. conceived the project and designed the experiments. L.P.G and H.Z. were responsible for the collection, analysis of overall experimental data, the interpretation of the results and revised the manuscript. J.M.C., Y.J.G., J.Y.M., D.T.C. and Z.Y.L. contributed to data collection of behavioral tests. C.Y.W., B.L., X.Q.Z., G.H.L. and H.W.S. contributed to data interpretation and manuscript editing; L.S. contributed to editing as well as final approval of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This work was carried out based on the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China, with the approval of the Ethics Committee of Shandong Second Medical University (Approval No. 2021SDL203). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gou, L., Zheng, H., Chen, J. et al. Social hierarchy and resilience affect stress-induced PTSD via Uba7 gene expression and subsequent inflammation in microglia of the mPFC. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03171-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03171-1