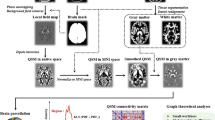
Abstract
Mild cognitive impairment (MCI), the prodromal stage of dementia, is characterized by cognitive dysfunction and white matter (WM) disruption. To investigate the WM microstructure degeneration at different scales in MCI, the orientation-sensitive fixel-based analysis was performed with fixelwise, fiberwise fiber-specific measures in 50 patients with MCI compared to 75 healthy controls. Moreover, to clarify the patterns of the organization operating at different scales in MCI, the potential mediated effects of structural connectome in networkwise on the associations between microstructure alterations of 24 anatomical tracts and cognitive dysfunction were analyzed. Compared to healthy controls, specific tracts were widely disrupted in fiberwise in patients with MCI, represented by the decrease of fiber density and cross-section in most commissural fibers and many association fibers, which was consistent with previous findings. The associations between WM microstructural degeneration and multi-domain cognitive dysfunction were also observed. Interestingly, the structural connectome between visual and salience networks played a potential mediating role in the relationship between disruption of WM microstructure and worse language performance, and we also found a similar situation in the memory domain. The present study provides mechanistic insight into the relationship between microstructure damage and cognitive dysfunction in prodromal dementia under a multilevel WM hierarchy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data and relevant code that support the findings of this study are available upon reasonable request to the corresponding author. ADNI data are available and can be searched from adni.loni.usc.edu after applying to the ADNI database.
References
Reuben DB, Kremen S, Maust DT. Dementia prevention and treatment: a narrative review. JAMA Intern Med. 2024;184:563–72.
Stephan BC, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17:1056–76.
Jack CR Jr., Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024;20:5143–69.
Brueggen K, Dyrba M, Cardenas-Blanco A, Schneider A, Fliessbach K, Buerger K, et al. Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease based on multicenter diffusion tensor imaging. J Neurol. 2019;266:2465–74.
Crous-Bou M, Minguillon C, Gramunt N, Molinuevo JL. Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther. 2017;9:71.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46.
Karim HT, Aizenstein HJ, Mizuno A, Ly M, Andreescu C, Wu M, et al. Independent replication of advanced brain age in mild cognitive impairment and dementia: detection of future cognitive dysfunction. Mol Psychiatry. 2022;27:5235–43.
Chen Y, Wang Y, Song Z, Fan Y, Gao T, Tang X. Abnormal white matter changes in Alzheimer’s disease based on diffusion tensor imaging: a systematic review. Ageing Res Rev. 2023;87:101911.
Meinert S, Nowack N, Grotegerd D, Repple J, Winter NR, Abheiden I, et al. Association of brain white matter microstructure with cognitive performance in major depressive disorder and healthy controls: a diffusion-tensor imaging study. Mol Psychiatry. 2022;27:1103–10.
Vanderlinden G, Ceccarini J, Vande Casteele T, Michiels L, Lemmens R, Triau E, et al. Spatial decrease of synaptic density in amnestic mild cognitive impairment follows the tau build-up pattern. Mol Psychiatry. 2022;27:4244–51.
Yan T, Wang W, Yang L, Chen K, Chen R, Han Y. Rich club disturbances of the human connectome from subjective cognitive decline to Alzheimer’s disease. Theranostics. 2018;8:3237–55.
Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, et al. White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement. 2012;8:S112–21.
Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34:2747–66.
Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 2011;55:880–90.
Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58–73.
Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, et al. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018;141:888–902.
Dhollander T, Clemente A, Singh M, Boonstra F, Civier O, Duque JD, et al. Fixel-based analysis of diffusion MRI: methods, applications, challenges and opportunities. Neuroimage. 2021;241:118417.
Roberts G, Perry A, Lord A, Frankland A, Leung V, Holmes-Preston E, et al. Structural dysconnectivity of key cognitive and emotional hubs in young people at high genetic risk for bipolar disorder. Mol Psychiatry. 2016;23:413–21.
Breakspear M. Dynamic models of large-scale brain activity. Nat Neurosci. 2017;20:340–52.
Maier-Hein, Neher KH, Houde PF, Cote MA JC, Garyfallidis E, Zhong J, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8:1349.
Vriend C, de Joode NT, Pouwels PJW, Liu F, Otaduy MCG, Pastorello B, et al. Age of onset of obsessive-compulsive disorder differentially affects white matter microstructure. Mol Psychiatry. 2024;29:1033–45.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s disease neuroimaging initiative (ADNI) clinical characterization. Neurology. 2010;74:201–9.
Mukherjee S, Choi S-E, Lee ML, Scollard P, Trittschuh EH, Mez J, et al. Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology. 2023;37:409–23.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci USA. 2000;97:11050–5.
Gaser C, Dahnke R, Thompson PM, Kurth F, Luders E, the Alzheimer’s Disease Neuroimaging. Initiative. CAT: a computational anatomy toolbox for the analysis of structural MRI data. GigaScience. 2024;13:giae049.
Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137.
Wasserthal J, Neher P, Maier-Hein KH. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage. 2018;183:239–53.
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–79.
Smith RE, Tournier JD, Calamante F, Connelly A. SIFT: spherical-deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298–312.
Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40–55.
Mak E, Gabel S, Mirette H, Su L, Williams GB, Waldman A, et al. Structural neuroimaging in preclinical dementia: from microstructural deficits and grey matter atrophy to macroscale connectomic changes. Ageing Res Rev. 2017;35:250–64.
Dewenter A, Jacob MA, Cai M, Gesierich B, Hager P, Kopczak A, et al. Disentangling the effects of Alzheimer’s and small vessel disease on white matter fibre tracts. Brain. 2023;146:678–89.
Lam B, Masellis M, Freedman M, Stuss DT, Black SE. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther. 2013;5:1.
Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–11.
Zarkali A, McColgan P, Leyland LA, Lees AJ, Rees G, Weil RS. Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction. Neurology. 2020;94:e1525–38.
Wang Q, Schindler SE, Chen G, McKay NS, McCullough A, Flores S, et al. Investigating white matter neuroinflammation in Alzheimer disease using diffusion-based neuroinflammation imaging. Neurology. 2024;102:e208013.
Fan LY, Lai YM, Chen TF, Hsu YC, Chen PY, Huang KZ, et al. Diminution of context association memory structure in subjects with subjective cognitive decline. Hum Brain Mapp. 2018;39:2549–62.
Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–8.
Nowrangi MA, Lyketsos CG, Leoutsakos JM, Oishi K, Albert M, Mori S, et al. Longitudinal, region-specific course of diffusion tensor imaging measures in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2013;9:519–28.
De Guio F, Mangin JF, Duering M, Ropele S, Chabriat H, Jouvent E. White matter edema at the early stage of cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2015;46:258–61.
Metzler-Baddeley C, Hunt S, Jones DK, Leemans A, Aggleton JP, O’Sullivan MJ. Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment. Neurology. 2012;79:2233–40.
Mito R, Vaughan DN, Semmelroch M, Connelly A, Jackson GD. Bilateral structural network abnormalities in epilepsy associated with bottom-of-sulcus dysplasia. Neurology. 2022;98:e152–63.
Shu N, Wang X, Bi Q, Zhao T, Han Y. Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology. 2018;286:229–38.
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–40.
Croll PH, Vernooij MW, Reid RI, Goedegebure A, Power MC, Rigters SC, et al. Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement. 2020;16:1515–23.
Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122:250–61.
Vaquero L, Rousseau PN, Vozian D, Klein D, Penhune V. What you learn & when you learn it: Impact of early bilingual & music experience on the structural characteristics of auditory-motor pathways. Neuroimage. 2020;213:116689.
Ghulam-Jelani Z, Barrios-Martinez J, Eguiluz-Melendez A, Gomez R, Anania Y, Yeh FC. Redundancy circuits of the commissural pathways in human and rhesus macaque brains. Hum Brain Mapp. 2021;42:2250–61.
Martino M, Magioncalda P. A three-dimensional model of neural activity and phenomenal-behavioral patterns. Mol Psychiatry. 2023;29:639–52.
Luo N, Sui J, Abrol A, Lin D, Chen J, Vergara VM, et al. Age-related structural and functional variations in 5,967 individuals across the adult lifespan. Hum Brain Mapp. 2020;41:1725–37.
Guptarak J, Scaduto P, Tumurbaatar B, Zhang WR, Jupiter D, Taglialatela G, et al. Cognitive integrity in non-demented individuals with Alzheimer’s neuropathology is associated with preservation and remodeling of dendritic spines. Alzheimers Dement. 2024;20:4677–91.
Fingerhut H, Gozdas E, Hosseini SMH. Quantitative MRI evidence for cognitive reserve in healthy elders and prodromal Alzheimer’s disease. J Alzheimers Dis. 2022;89:849–63.
Jiang R, Scheinost D, Zuo N, Wu J, Qi S, Liang Q, et al. A neuroimaging signature of cognitive aging from whole-brain functional connectivity. Adv Sci. 2022;9:e2201621.
Cho S, Olm CA, Ash S, Shellikeri S, Agmon G, Cousins KAQ, et al. Automatic classification of AD pathology in FTD phenotypes using natural speech. Alzheimers Dement. 2024;20:3416–28.
Albouy P, Martinez-Moreno ZE, Hoyer RS, Zatorre RJ, Baillet S. Supramodality of neural entrainment: rhythmic visual stimulation causally enhances auditory working memory performance. Sci Adv. 2022;8:eabj9782.
Liu T, Wang M, Zhang J, Ye C, Funahashi S, Liu J, et al. Brain network dynamics in patients with single- and multiple-domain amnestic mild cognitive impairment. Alzheimers Dement. 2024;20:7657–74.
Yao D, Calhoun VD, Fu Z, Du Y, Sui J. An ensemble learning system for a 4-way classification of Alzheimer’s disease and mild cognitive impairment. J Neurosci Methods. 2018;302:75–81.
Dong Y, Wang S, Huang Q, Berg RW, Li G, He J. Neural decoding for intracortical brain-computer interfaces. Cyborg Bionic Syst. 2023;4:0044.
Yeh CH, Zhang C, Shi W, Lo MT, Tinkhauser G, Oswal A. Cross-frequency coupling and intelligent neuromodulation. Cyborg Bionic Syst. 2023;4:0034.
Acknowledgements
Thank Konstantinos Ioannou and Konstantinos Chiotis from Karolinska Institutet for their assistance in formatting. Data collection and sharing for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) is funded by the National Institute on Aging (National Institutes of Health Grant U19AG024904). The grantee organization is the Northern California Institute for Research and Education. In the past, ADNI has also received funding from the National Institute of Biomedical Imaging and Bioengineering, the Canadian Institutes of Health Research, and private sector contributions through the Foundation for the National Institutes of Health (FNIH) including generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics.
Funding
This work was supported by STI2030-Major Projects (2022ZD0208500), Key-Area Research and Development Program of Guangdong Province (2023B0303030002), the National Natural Science Foundation of China (grant numbers U20A20191, 62336002, 62306035, 62373056, 62406025), the Beijing Natural Science Foundation (IS23115).
Author information
Authors and Affiliations
Consortia
Contributions
TY, YC, JZ and TL contributed to the conception and design of the work; TY, YC, TL, LC and JZ analyzed data, interpreted results of experiments and prepared figures; TY, YC, JZ, TL and LW drafted and revised the manuscript; SF, XT, JW and WM provided study supervision. Although the investigators for ADNI contributed to providing data, they did not participate in the analysis or writing of this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Data acquisition of ADNI was conducted in compliance with the protocol (NCT02854033), in accordance with GCP guidelines, all relevant regulations and laws. All subjects were informed about the aims of the study and signed a consent form under the approval of the Institutional Review Boards (IRB) and Research Ethics Boards (REB). More details on ethics approval and consent to participate in ADNI can be found at https://adni.loni.usc.edu/.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Liu, T., Cao, LZ. et al. Multilevel white matter degeneration associated cognitive dysfunction in mild cognitive impairment. Mol Psychiatry 31, 835–844 (2026). https://doi.org/10.1038/s41380-025-03179-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03179-7