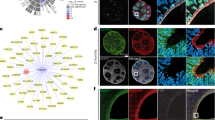
Abstract
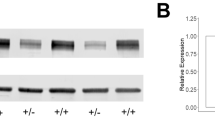
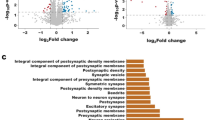
The Ras GTPase-activating protein SynGAP interacts with PSD95 to regulate synaptic morphology and function at the postsynaptic density in neurons. Haploinsufficiency of SYNGAP1 has been linked to autism spectrum disorders (ASD) and intellectual disability (ID). While transcriptional and translational regulation of SYNGAP1 has been extensively explored, the mechanisms governing its protein homeostasis remain largely elusive. In this study, we discovered that Necdin, a protein linked to Prader-Willi syndrome (PWS), interacts with SynGAP and regulates its stability through the SGT1-HSP90 chaperone machinery; notably, depletion of Necdin results in decreased SynGAP protein levels in mice. Loss of Necdin lead to impaired sociability, accompanied by an increased number of dendritic spines and a higher proportion of mature spines in pyramidal neurons of the medial prefrontal cortex (mPFC) in mice. Electrophysiological recordings revealed elevated frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs) and reduced amplitude of miniature inhibitory postsynaptic currents (mIPSCs) in these neurons. Targeted viral overexpression of Syngap1 in the mPFC of Necdin-deficient mice rescued the deficits in sociability, synaptic function, and dendritic spine morphology. Collectively, our findings reveal Necdin as a key regulator of SynGAP protein homeostasis and highlight the contribution of post-translational regulation in the pathogenesis of ASD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The IP-MS datasets generated in this study have been deposited in two public repositories: the ProteomeXchange Consortium (PXD064040) and the iProX database (IPX0011995000). All other raw data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Grafodatskaya D, Chung B, Szatmari P, Weksberg R. Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry. 2010;49:794–809.
Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–16.
Shen MD, Swanson MR, Wolff JJ, Elison JT, Girault JB, Kim SH, et al. Subcortical brain development in autism and fragile x syndrome: evidence for dynamic, age- and disorder-specific trajectories in infancy. Am J Psychiatry. 2022;179:562–72.
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10–26.
Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38:1249–63.
Dykens EM, Lee E, Roof E. Prader-Willi syndrome and autism spectrum disorders: an evolving story. J Neurodev Disord. 2011;3:225–37.
Genovese AC, Butler MG. Behavioral and psychiatric disorders in syndromic autism. Brain Sciences. 2024;14:343.
Zhang J, Cai F, Lu R, Xing X, Xu L, Wu K, et al. CNTNAP2 intracellular domain (CICD) generated by gamma-secretase cleavage improves autism-related behaviors. Signal Transduct Target Ther. 2023;8:219.
Tamada K, Fukumoto K, Toya T, Nakai N, Awasthi JR, Tanaka S, et al. Genetic dissection identifies Necdin as a driver gene in a mouse model of paternal 15q duplications. Nat Commun. 2021;12:4056.
Yoshikawa K. Necdin: A purposive integrator of molecular interaction networks for mammalian neuron vitality. Genes Cells. 2021;26:641–83.
Maruyama K, Usami M, Aizawa T, Yoshikawa K. A novel brain-specific mRNA encoding nuclear protein (necdin) expressed in neurally differentiated embryonal carcinoma cells. Biochem Biophys Res Commun. 1991;178:291–6.
Aizawa T, Maruyama K, Kondo H, Yoshikawa K. Expression of necdin, an embryonal carcinoma-derived nuclear protein, in developing mouse brain. Brain Res Dev Brain Res. 1992;68:265–74.
Hayashi Y, Matsuyama K, Takagi K, Sugiura H, Yoshikawa K. Arrest of cell growth by necdin, a nuclear protein expressed in postmitotic neurons. Biochem Biophys Res Commun. 1995;213:317–24.
Matarazzo V, Caccialupi L, Schaller F, Shvarev Y, Kourdougli N, Bertoni A, et al. Necdin shapes serotonergic development and SERT activity modulating breathing in a mouse model for Prader-Willi syndrome. Elife. 2017;6:e32640.
Kuwako K, Taniura H, Yoshikawa K. Necdin-related MAGE proteins differentially interact with the E2F1 transcription factor and the p75 neurotrophin receptor. J Biol Chem. 2004;279:1703–12.
Minamide R, Fujiwara K, Hasegawa K, Yoshikawa K. Antagonistic interplay between necdin and Bmi1 controls proliferation of neural precursor cells in the embryonic mouse neocortex. PLoS One. 2014;9:e84460.
Hasegawa K, Yasuda T, Shiraishi C, Fujiwara K, Przedborski S, Mochizuki H, et al. Promotion of mitochondrial biogenesis by necdin protects neurons against mitochondrial insults. Nat Commun. 2016;7:10943.
Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28:8772–84.
Lu R, Dong Y, Li J-D. Necdin regulates BMAL1 stability and circadian clock through SGT1-HSP90 chaperone machinery. Nucleic Acids Res. 2020;48:7944–57.
Liu D, Xie Z, Gu P, Li X, Zhang Y, Wang X, et al. Cry1Delta11 mutation induces ADHD-like symptoms through hyperactive dopamine D1 receptor signaling. JCI Insight. 2023;8:e170434.
Gao Q, Tian R, Han H, Slone J, Wang C, Ke X, et al. PINK1-mediated Drp1(S616) phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission. Signal Transduct Target Ther. 2022;7:103.
Viesselmann C, Ballweg J, Lumbard D, Dent EW Nucleofection and primary culture of embryonic mouse hippocampal and cortical neurons. J Vis Exp 2011, 2373.
Muscatelli F, Abrous DN, Massacrier A, Boccaccio I, Le Moal M, Cau P, et al. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum Mol Genet. 2000;9:3101–10.
Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94.
Bian WJ, Miao WY, He SJ, Qiu Z, Yu X. Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell. 2015;162:808–22.
Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–40.
Stephens DJ, Banting G. In vivo dynamics of the F-actin-binding protein neurabin-II. Biochem J. 2000;345:185–94.
Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–82.
Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–7.
Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–81.
Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–32.
Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momenan R, et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am J Psychiatry. 2010;167:1508–17.
Choi L, An JY. Genetic architecture of autism spectrum disorder: Lessons from large-scale genomic studies. Neurosci Biobehav Rev. 2021;128:244–57.
Uchino S, Waga C. SHANK3 as an autism spectrum disorder-associated gene. Brain Dev. 2013;35:106–10.
Berryer MH, Hamdan FF, Klitten LL, Moller RS, Carmant L, Schwartzentruber J, et al. Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Hum Mutat. 2013;34:385–94.
Vazquez LE, Chen HJ, Sokolova I, Knuesel I, Kennedy MB. SynGAP regulates spine formation. J Neurosci. 2004;24:8862–72.
Nakajima R, Takao K, Hattori S, Shoji H, Komiyama NH, Grant SGN, et al. Comprehensive behavioral analysis of heterozygous Syngap1 knockout mice. Neuropsychopharmacol Rep. 2019;39:223–37.
Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66:771–82.
Na ES, Nelson ED, Kavalali ET, Monteggia LM. The impact of MeCP2 loss- or gain-of-function on synaptic plasticity. Neuropsychopharmacology. 2013;38:212–9.
Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, et al. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. J Neurosci. 2012;32:3109–17.
Zoghbi HY. MeCP2 dysfunction in humans and mice. J Child Neurol. 2005;20:736–40.
Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, et al. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–57.
Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–53.
Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–4.
Segal I, Korkotian I, Murphy DD. Dendritic spine formation and pruning: common cellular mechanisms? Trends Neurosci. 2000;23:53–57.
Chen M, Qi J, Poo M, Yang Y. Stability and dynamics of dendritic spines in macaque prefrontal cortex. Natl Sci Rev. 2022;9:nwac125.
Chen Y, Wang Y, Erturk A, Kallop D, Jiang Z, Weimer RM, et al. Activity-induced Nr4a1 regulates spine density and distribution pattern of excitatory synapses in pyramidal neurons. Neuron. 2014;83:431–43.
Phillips M, Pozzo-Miller L. Dendritic spine dysgenesis in autism related disorders. Neurosci Lett. 2015;601:30–40.
Wang M, Li H, Takumi T, Qiu Z, Xu X, Yu X, et al. Distinct Defects in Spine Formation or Pruning in Two Gene Duplication Mouse Models of Autism. Neurosci Bull. 2017;33:143–52.
Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci USA. 2006;103:4344–51.
Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904.
Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–91.
Su P, Lai TKY, Lee FHF, Abela AR, Fletcher PJ, Liu F. Disruption of SynGAP-dopamine D1 receptor complexes alters actin and microtubule dynamics and impairs GABAergic interneuron migration. Sci Signal. 2019;12:eaau9122.
Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28:13673–83.
Clement JP, Aceti M, Creson TK, Ozkan ED, Shi Y, Reish NJ, et al. Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses. Cell. 2012;151:709–23.
Barnes SA, Wijetunge LS, Jackson AD, Katsanevaki D, Osterweil EK, Komiyama NH, et al. Convergence of Hippocampal Pathophysiology in Syngap+/- and Fmr1-/y Mice. J Neurosci. 2015;35:15073–81.
Nonaka M, Doi T, Fujiyoshi Y, Takemoto-Kimura S, Bito H. Essential contribution of the ligand-binding beta B/beta C loop of PDZ1 and PDZ2 in the regulation of postsynaptic clustering, scaffolding, and localization of postsynaptic density-95. J Neurosci. 2006;26:763–74.
McMahon AC, Barnett MW, O’Leary TS, Stoney PN, Collins MO, Papadia S, et al. SynGAP isoforms exert opposing effects on synaptic strength. Nat Commun. 2012;3:900.
Yang R, Feng X, Arias-Cavieres A, Mitchell RM, Polo A, Hu K, et al. Upregulation of SYNGAP1 expression in mice and human neurons by redirecting alternative splicing. Neuron. 2023;111:1637–1650.e1635.
Araki Y, Hong I, Gamache TR, Ju S, Collado-Torres L, Shin JH, et al. SynGAP isoforms differentially regulate synaptic plasticity and dendritic development. Elife. 2020;9:e56273.
Yokoi S, Udagawa T, Fujioka Y, Honda D, Okado H, Watanabe H, et al. 3’UTR Length-Dependent control of SynGAP isoform alpha2 mRNA by FUS and ELAV-like proteins promotes dendritic spine maturation and cognitive function. Cell Rep. 2017;20:3071–84.
Lai KO, Jordan BA, Ma XM, Srivastava DP, Tolias KF. Molecular mechanisms of dendritic spine development and plasticity. Neural Plast. 2016;2016:2078121.
Ebrahimi-Fakhari D, Sahin M. Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr Opin Neurol. 2015;28:91–102.
Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–27.
Sidorov MS, Auerbach BD, Bear MF. Fragile X mental retardation protein and synaptic plasticity. Mol Brain. 2013;6:15.
Carver JA, Ecroyd H, Truscott RJW, Thorn DC, Holt C. Proteostasis and the regulation of intra- and extracellular protein aggregation by ATP-independent molecular chaperones: lens alpha-crystallins and milk caseins. Acc Chem Res. 2018;51:745–52.
Wyatt AR, Yerbury JJ, Ecroyd H, Wilson MR. Extracellular chaperones and proteostasis. Annu Rev Biochem. 2013;82:295–322.
Chamberlain LH, Burgoyne RD. Cysteine-string protein: the chaperone at the synapse. J Neurochem. 2000;74:1781–9.
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–7.
Kim JK, Jha NN, Awano T, Caine C, Gollapalli K, Welby E, et al. A spinal muscular atrophy modifier implicates the SMN protein in SNARE complex assembly at neuromuscular synapses. Neuron. 2023;111:1423–1439.e1424.
Gerges NZ, Tran IC, Backos DS, Harrell JM, Chinkers M, Pratt WB, et al. Independent functions of hsp90 in neurotransmitter release and in the continuous synaptic cycling of AMPA receptors. J Neurosci. 2004;24:4758–66.
Chen Y, Wang B, Liu D, Li JJ, Xue Y, Sakata K, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci. 2014;34:2464–70.
Acknowledgements
This project is funded by the National Natural Science Foundation of China (32371218, 31972913, 82070815, 32400690); Hunan Provincial grants (2021DK2001, 2023SK2084, 2023RC4001, 2024JJ5540, 2025DK2004, 2025JJ30041, 2025JJ60134); Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” (2018B030335001).
Author information
Authors and Affiliations
Contributions
J.Z., H.L. and J-D.L. conceived the experiments. X.L. performed the behavioral experiments, spine morphology and SynGAP rescuing experiments. X.L. and I.B. performed the electrophysiology analysis under the supervision of Y.S. and S.D.. X.L., R.L. and D.L. performed the RNA-seq and IP-MS analysis under the supervision of Z.C.. J.Z. performed the Necdin-SynGAP interaction and influence of Necdin-SGT1-HSP90 on the stability of SynGAP. J-D.L., J.Z. and H.L. supervised the project. X.L. J.Z. and J-D.L. wrote and revised the manuscript with contributions from all authors. H.L. contributed to the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the relevant guidelines and regulations. All animal experiments were approved by the Ethics Committee of the School of Life Sciences, Central South University, China (Approval No. 2019-2-7). Consent to participate is not applicable as this study did not involve human participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Bader, I., Li, X. et al. Loss of Necdin causes social deficit and aberrant synaptic function through destabilization of SynGAP. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03187-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03187-7