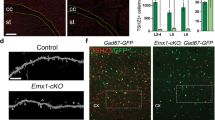
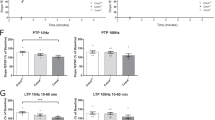
Abstract
Repetitive behaviors are cardinal features of many brain disorders, including autism spectrum disorder (ASD). We previously associated dysfunction of striatal cholinergic interneurons (SCINs) with repetitive behaviors in a mouse model based on conditional deletion of the ASD-related gene Tshz3 in cholinergic neurons (Chat-cKO). Here, we provide evidence linking SCIN abnormalities to the unique organization of the striatum into striosome and matrix compartments, whose imbalances are implicated in several pathological conditions. Chat-cKO mice exhibit an altered relationship between the embryonic birthdate of SCINs and their adult striosome-matrix distribution, leading to an increased proportion of striosomal SCINs. In addition, the ratio of striosomal SCINs with slow-irregular vs. sustained-regular firing is increased, which translates into decreased activity, further stressing the striosome-matrix imbalance. These findings provide novel insights into the pathogenesis of ASD-related stereotyped behaviors by pointing to abnormal developmental compartmentalization and activity of SCINs as a substrate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw data sets will be made available upon reasonable request to the corresponding authors.
References
Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed., Arlington (VA): American Psychiatric Association; 2013.
Fuccillo MV. Striatal circuits as a common node for autism pathophysiology. Front Neurosci. 2016;10:27.
Li W, Pozzo-Miller L. Dysfunction of the corticostriatal pathway in autism spectrum disorders. J Neurosci Res. 2019;98:2130–47.
Rapanelli M, Frick LR, Pittenger C. The role of interneurons in autism and tourette syndrome. Trends Neurosci. 2017;40:397–407.
Contractor A, Ethell IM, Portera-Cailliau C. Cortical interneurons in autism. Nat Neurosci. 2021;24:1648–59.
Ridley RM. The psychology of perserverative and stereotyped behaviour. Prog Neurobiol. 1994;44:221–31.
Jutla A, Foss-Feig J, Veenstra-VanderWeele J. Autism spectrum disorder and schizophrenia: an updated conceptual review. Autism Res. 2022;15:384–412.
Figee M, Pattij T, Willuhn I, Luigjes J, van den Brink W, Goudriaan A, et al. Compulsivity in obsessive-compulsive disorder and addictions. Eur Neuropsychopharmacol. 2016;26:856–68.
Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–92.
Burton CL, Longaretti A, Zlatanovic A, Gomes GM, Tonini R. Striatal insights: a cellular and molecular perspective on repetitive behaviors in pathology. Front Cell Neurosci. 2024;18:1386715.
Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91.
Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in tourette syndrome. Biol Psychiatry. 2016;79:372–82.
Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA. 2015;112:893–8.
Martos YV, Braz BY, Beccaria JP, Murer MG, Belforte JE. Compulsive social behavior emerges after selective ablation of striatal cholinergic interneurons. J Neurosci. 2017;37:2849–58.
Xu J, Liu RJ, Fahey S, Frick L, Leckman J, Vaccarino F, et al. Antibodies from children with PANDAS bind specifically to striatal cholinergic interneurons and alter their activity. Am J Psychiatry. 2021;178:48–64.
Aliane V, Perez S, Bohren Y, Deniau JM, Kemel ML. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134:110–8.
Crittenden JR, Lacey CJ, Weng FJ, Garrison CE, Gibson DJ, Lin Y, et al. Striatal cholinergic interneurons modulate spike-timing in striosomes and matrix by an amphetamine-sensitive mechanism. Front Neuroanat. 2017;11:20.
Prado VF, Janickova H, Al-Onaizi MA, Prado MA. Cholinergic circuits in cognitive flexibility. Neuroscience. 2017;345:130–41.
Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. 2019;49:604–22.
Apicella P. The role of the intrinsic cholinergic system of the striatum: what have we learned from TAN recordings in behaving animals? Neuroscience. 2017;360:81–94.
Goldberg JA, Wilson CJ. The cholinergic interneurons of the striatum: intrinsic properties underlie multiple discharge patterns. In: Steiner H, Tseng KY, editors. Handbook of basal ganglia structure and function. London (UK): Academic Press; 2010, pp 133–49.
Ahmed NY, Knowles R, Dehorter N. New insights into cholinergic neuron diversity. Front Mol Neurosci. 2019;12:204.
Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–6.
Brimblecombe KR, Cragg SJ. The striosome and matrix compartments of the striatum: a path through the labyrinth from neurochemistry toward function. ACS Chem Neurosci. 2017;8:235–42.
Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8.
Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–9.
Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59.
Graybiel AM, Matsushima A. Striosomes and matrisomes: scaffolds for dynamic coupling of volition and action. Annu Rev Neurosci. 2023;46:359–80.
Lazaridis I, Crittenden JR, Ahn G, Hirokane K, Wickersham IR, Yoshida T, et al. Striosomes control dopamine via dual pathways paralleling canonical basal ganglia circuits. Curr Biol. 2024;34:5263–83.e8.
Kuo HY, Liu FC. Pathological alterations in striatal compartments in the human brain of autism spectrum disorder. Mol Brain. 2020;13:83.
Kuo HY, Liu FC. Valproic acid induces aberrant development of striatal compartments and corticostriatal pathways in a mouse model of autism spectrum disorder. FASEB J. 2017;31:4458–71.
Murray RC, Logan MC, Horner KA. Striatal patch compartment lesions reduce stereotypy following repeated cocaine administration. Brain Res. 2015;1618:286–98.
Caubit X, Gubellini P, Andrieux J, Roubertoux PL, Metwaly M, Jacq B, et al. TSHZ3 deletion causes an autism syndrome and defects in cortical projection neurons. Nat Genet. 2016;48:1359–69.
Caubit X, Gubellini P, Roubertoux PL, Carlier M, Molitor J, Chabbert D, et al. Targeted Tshz3 deletion in corticostriatal circuit components segregates core autistic behaviors. Transl Psychiatry. 2022;12:106.
Caubit X, Arbeille E, Chabbert D, Desprez F, Messak I, Fatmi A, et al. Camk2a-Cre and Tshz3 expression in mouse striatal cholinergic interneurons: implications for autism spectrum disorder. Front Genet. 2021;12:683959.
Chabbert D, Caubit X, Roubertoux PL, Carlier M, Habermann B, Jacq B, et al. Postnatal Tshz3 deletion drives altered corticostriatal function and autism spectrum disorder-like behavior. Biol Psychiatry. 2019;86:274–85.
Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–9.
Roubertoux PL, Tordjman S, Caubit X, di Cristopharo J, Ghata A, Fasano L, et al. Construct validity and cross validity of a test battery modeling Autism Spectrum Disorder (ASD) in mice. Behav Genet. 2020;50:26–40.
Knowles R, Dehorter N, Ellender T. From progenitors to progeny: shaping striatal circuit development and function. J Neurosci. 2021;41:9483–502.
Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–76.
Pappas SS, Li J, LeWitt TM, Kim JK, Monani UR, Dauer WT. A cell autonomous torsinA requirement for cholinergic neuron survival and motor control. Elife. 2018;7:e36691.
Lopes R, Verhey van Wijk N, Neves G, Pachnis V. Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc Natl Acad Sci USA. 2012;109:3119–24.
Furusho M, Ono K, Takebayashi H, Masahira N, Kagawa T, Ikeda K, et al. Involvement of the Olig2 transcription factor in cholinergic neuron development of the basal forebrain. Dev Biol. 2006;293:348–57.
Caubit X, Tiveron MC, Cremer H, Fasano L. Expression patterns of the three Teashirt-related genes define specific boundaries in the developing and postnatal mouse forebrain. J Comp Neurol. 2005;486:76–88.
van Vulpen EH, van der Kooy D. Striatal cholinergic interneurons: birthdates predict compartmental localization. Brain Res Dev Brain Res. 1998;109:51–8.
Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52.
Rusu SI, Pennartz CMA. Learning, memory and consolidation mechanisms for behavioral control in hierarchically organized cortico-basal ganglia systems. Hippocampus. 2020;30:73–98.
Song MR, Lee SW. Rethinking dopamine-guided action sequence learning. Eur J Neurosci. 2024;60:3447–65.
Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–32.
Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–85.
Zhao Z, Zhang K, Liu X, Yan H, Ma X, Zhang S, et al. Involvement of HCN channel in muscarinic inhibitory action on tonic firing of dorsolateral striatal cholinergic interneurons. Front Cell Neurosci. 2016;10:71.
Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–96.
Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–19.
Prado VF, Roy A, Kolisnyk B, Gros R, Prado MA. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 2013;450:265–74.
Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, et al. Altered baseline and nicotine-mediated behavioral and cholinergic profiles in ChAT-Cre mouse lines. J Neurosci. 2018;38:2177–88.
Hirsch EC, Graybiel AM, Hersh LB, Duyckaerts C, Agid Y. Striosomes and extrastriosomal matrix contain different amounts of immunoreactive choline acetyltransferase in the human striatum. Neurosci Lett. 1989;96:145–50.
Graybiel AM, Baughman RW, Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986;323:625–7.
Van Zandt M, Flanagan D, Pittenger C. Sex differences in the distribution and density of regulatory interneurons in the striatum. Front Cell Neurosci. 2024;18:1415015.
Unal B, Ibanez-Sandoval O, Shah F, Abercrombie ED, Tepper JM. Distribution of tyrosine hydroxylase-expressing interneurons with respect to anatomical organization of the neostriatum. Front Syst Neurosci. 2011;5:41.
Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–83.
Kovesdi E, Udvaracz I, Kecskes A, Szocs S, Farkas S, Faludi P, et al. 17beta-estradiol does not have a direct effect on the function of striatal cholinergic interneurons in adult mice in vitro. Front Endocrinol (Lausanne). 2022;13:993552.
van der Kooy D, Fishell G. Neuronal birthdate underlies the development of striatal compartments. Brain Res. 1987;401:155–61.
Song DD, Harlan RE. Genesis and migration patterns of neurons forming the patch and matrix compartments of the rat striatum. Brain Res Dev Brain Res. 1994;83:233–45.
Graybiel AM, Hickey TL. Chemospecificity of ontogenetic units in the striatum: demonstration by combining [3H]thymidine neuronography and histochemical staining. Proc Natl Acad Sci USA. 1982;79:198–202.
Lebouc M, Richard Q, Garret M, Baufreton J. Striatal circuit development and its alterations in Huntington’s disease. Neurobiol Dis. 2020;145:105076.
Chen L, Chatterjee M, Li JY. The mouse homeobox gene Gbx2 is required for the development of cholinergic interneurons in the striatum. J Neurosci. 2010;30:14824–34.
Poppi LA, Ho-Nguyen KT, Shi A, Daut CT, Tischfield MA. Recurrent implication of striatal cholinergic interneurons in a range of neurodevelopmental, neurodegenerative, and neuropsychiatric disorders. Cells. 2021;10:907.
Crittenden JR, Lacey CJ, Lee T, Bowden HA, Graybiel AM. Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice. Front Neural Circuits. 2014;8:57.
Kubota Y, Kawaguchi Y. Spatial distributions of chemically identified intrinsic neurons in relation to patch and matrix compartments of rat neostriatum. J Comp Neurol. 1993;332:499–513.
Johnston JG, Gerfen CR, Haber SN, van der Kooy D. Mechanisms of striatal pattern formation: conservation of mammalian compartmentalization. Brain Res Dev Brain Res. 1990;57:93–102.
Lee H, Leamey CA, Sawatari A. Rapid reversal of chondroitin sulfate proteoglycan associated staining in subcompartments of mouse neostriatum during the emergence of behaviour. PLoS One. 2008;3:e3020.
Martone ME, Young SJ, Armstrong DM, Groves PM. The distribution of cholinergic perikarya with respect to enkephalin-rich patches in the caudate nucleus of the adult cat. J Chem Neuroanat. 1994;8:47–59.
Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–503.
Lozovaya N, Eftekhari S, Cloarec R, Gouty-Colomer LA, Dufour A, Riffault B, et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat Commun. 2018;9:1422.
Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–23.
Marotta R, Risoleo MC, Messina G, Parisi L, Carotenuto M, Vetri L, et al. The neurochemistry of autism. Brain Sci. 2020;10:163.
Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology. 2014;39:831–40.
Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, Tamamaki N, et al. Targeted interneuron depletion in the dorsal striatum produces autism-like behavioral abnormalities in male but not female mice. Biol Psychiatry. 2017;82:194–203.
Athnaiel O, Job GA, Ocampo R, Teneqexhi P, Messer WS, Ragozzino ME. Effects of the partial M1 muscarinic cholinergic receptor agonist CDD-0102A on stereotyped motor behaviors and reversal learning in the BTBR mouse model of autism. Int J Neuropsychopharmacol. 2022;25:64–74.
Ferhat AT, Verpy E, Biton A, Forget B, De Chaumont F, Mueller F, et al. Excessive self-grooming, gene dysregulation and imbalance between the striosome and matrix compartments in the striatum of Shank3 mutant mice. Front Mol Neurosci. 2023;16:1139118.
Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–83.
Sako W, Morigaki R, Nagahiro S, Kaji R, Goto S. Olfactory type G-protein alpha subunit in striosome-matrix dopamine systems in adult mice. Neuroscience. 2010;170:497–502.
Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–65.
Peter Z, Oliphant ME, Fernandez TV. Motor stereotypies: a pathophysiological review. Front Neurosci. 2017;11:171.
Magno L, Barry C, Schmidt-Hieber C, Theodotou P, Hausser M, Kessaris N. NKX2-1 is required in the embryonic septum for cholinergic system development, learning, and memory. Cell Rep. 2017;20:1572–84.
Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136:3841–51.
Sreenivasan V, Serafeimidou-Pouliou E, Exposito-Alonso D, Bercsenyi K, Bernard C, Bae SE, et al. Input-specific control of interneuron numbers in nascent striatal networks. Proc Natl Acad Sci USA. 2022;119:e2118430119.
Fino E, Vandecasteele M, Perez S, Saudou F, Venance L. Region-specific and state-dependent action of striatal GABAergic interneurons. Nat Commun. 2018;9:3339.
Choi SJ, Ma TC, Ding Y, Cheung T, Joshi N, Sulzer D, et al. Alterations in the intrinsic properties of striatal cholinergic interneurons after dopamine lesion and chronic L-DOPA. Elife. 2020;9:e56920.
Cheng J, Umschweif G, Leung J, Sagi Y, Greengard P. HCN2 Channels in Cholinergic Interneurons of Nucleus Accumbens Shell Regulate Depressive Behaviors. Neuron. 2019;101:662–72.e5.
Ahmed NY, Knowles R, Liu L, Yan Y, Li X, Schumann U, et al. Developmental deficits of MGE-derived interneurons in the Cntnap2 knockout mouse model of autism spectrum disorder. Front Cell Dev Biol. 2023;11:1112062.
Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, et al. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6.
Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–24.
Inoue R, Suzuki T, Nishimura K, Miura M. Nicotinic acetylcholine receptor-mediated GABAergic inputs to cholinergic interneurons in the striosomes and the matrix compartments of the mouse striatum. Neuropharmacology. 2016;105:318–28.
Friedman A, Hueske E, Drammis SM, Toro Arana SE, Nelson ED, Carter CW, et al. Striosomes mediate value-based learning vulnerable in age and a Huntington’s disease model. Cell. 2020;183:918–34.e49.
Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, et al. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–10.
Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–42.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40.
Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204.
Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–35.
Haghdoust H, Janahmadi M, Behzadi G. Physiological role of dendrotoxin-sensitive K+ channels in the rat cerebellar Purkinje neurons. Physiol Res. 2007;56:807–13.
Maisano X, Litvina E, Tagliatela S, Aaron GB, Grabel LB, Naegele JR. Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci. 2012;32:46–61.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates, 2nd Edition. ed., San Diego (CA): Academic Press; 2001.
Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–3.
Acknowledgements
We wish to thank the imaging facility at the IBDM, member of the National Infrastructure France-BioImaging (https://ror.org/01y7vt929) supported by the French National Research Agency (ANR-24-INBS-0005 FBI BIOGEN), and the IBDM mouse facility.
Funding
This work received support from the French government under the Programme “Investissements d’Avenir”, Initiative d’Excellence d’Aix-Marseille Université via A*Midex funding (NeuroMarseille Institute, AMX-19-IET-004; MarMaRa Institute, AMX-19-IET-007), the French National Research Agency (ANR) “TSHZ3inASD” project grant n°ANR-17-CE16-0030-01 (to LF and LK-LG), the Fédération pour la Recherche sur le Cerveau (to LF), the Centre National de la Recherche Scientifique (CNRS), and Aix-Marseille University. JM and JG were supported by PhD grants from the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation.
Author information
Authors and Affiliations
Contributions
JM, JG, PS and XC performed the histological experiments and the quantitative analyses. JM and PG performed the patch-clamp experiments and analyzed the electrophysiological data. JM, JG and XC generated and maintained the transgenic mouse lines. FC performed western blot experiments. AF performed the qRT-PCR experiments and analyzed the data. JM, JG, XC and AF performed mouse genotyping. LK-LG, LF, XC and PG conceived the project, supervised the work and wrote the paper. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molitor, J., Graniou, J., Salin, P. et al. Altered striosome-matrix distribution and activity of striatal cholinergic interneurons in a model of autism-linked repetitive behaviors. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03208-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03208-5