Abstract
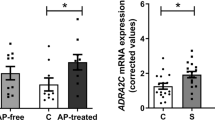
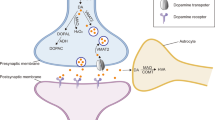
Prior work in animal models implicates abnormalities of adenosine metabolism in astrocytes as a possible pathophysiological mechanism underlying the symptoms of schizophrenia. In the present study, we sought to reverse-translate these findings back to the human brain in schizophrenia, focusing on the following questions: (1) Which components of the adenosine system are dysregulated in schizophrenia, and (2) are these changes limited to astrocytes? To address these questions, we captured enriched populations of DLPFC pyramidal neurons and astrocytes from schizophrenia and control subjects using laser capture microdissection and assessed expression of adenosine system components using qPCR. Interestingly, we found changes in enriched populations of astrocytes and neurons spanning metabolic and catabolic pathways. Ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD1) and ENTPD2 mRNA levels were significantly decreased (p < 0.05, n = 16 per group) in enriched populations of astrocytes; in pyramidal neurons equilibrative nucleoside transporter 1 (ENT1) and adenosine A1 receptor mRNA levels were significantly decreased, with an increase in adenosine deaminase (ADA) (p < 0.05, n = 16 per group). Rodent studies suggest that some of our findings (A1R and ENTPD2) may be due to treatment with antipsychotics. Our findings suggest changes in expression of genes involved in regulating metabolism of ATP in enriched populations of astrocytes, leading to lower availability of substrates needed to generate adenosine. In pyramidal neurons, changes in ENT1 and ADA mRNA may suggest increased catabolism of adenosine. These results offer new insights into the cell-subtype-specific pathophysiology of the adenosine system in this illness.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–49.
van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45.
Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 1982;39:991–7.
Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33.
Lara DR, Dall’Igna OP, Ghisolfi ES, Brunstein MG. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:617–29.
Ipata PL, Camici M, Micheli V, Tozz MG. Metabolic network of nucleosides in the brain. Curr Top Med Chem. 2011;11:909–22.
Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. 2016;139:1019–55.
Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26:3237–52.
Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia–opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–43.
Boison D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev. 2013;65:906–43.
Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–82.
Brunstein MG, Ghisolfi ES, Ramos FL, Lara DR. A clinical trial of adjuvant allopurinol therapy for moderately refractory schizophrenia. J Clin Psychiatry. 2005;66:213–9.
Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry. 2013;3:e321.
Deckert J, Brenner M, Durany N, Zochling R, Paulus W, Ransmayr G, et al. Up-regulation of striatal adenosine A(2A) receptors in schizophrenia. Neuroreport. 2003;14:313–6.
Kurumaji A, Toru M. An increase in [3H] CGS21680 binding in the striatum of postmortem brains of chronic schizophrenics. Brain Res. 1998;808:320–3.
Villar-Menendez I, Diaz-Sanchez S, Blanch M, Albasanz JL, Pereira-Veiga T, Monje A, et al. Reduced striatal adenosine A2A receptor levels define a molecular subgroup in schizophrenia. J Psychiatr Res. 2014;51:49–59.
Aliagas E, Villar-Menendez I, Sevigny J, Roca M, Romeu M, Ferrer I, et al. Reduced striatal ecto-nucleotidase activity in schizophrenia patients supports the “adenosine hypothesis”. Purinergic Signal. 2013;9:599–608.
Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6.
Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72.
Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–84.
Brundege JM, Dunwiddie TV. Metabolic regulation of endogenous adenosine release from single neurons. Neuroreport. 1998;9:3007–11.
Klyuch BP, Dale N, Wall MJ. Deletion of ecto-5’-nucleotidase (CD73) reveals direct action potential-dependent adenosine release. J Neurosci: Off J Soc Neurosci. 2012;32:3842–7.
Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA. 2012;109:6265–70.
McCullumsmith RE, O’Donovan SM, Drummond JB, Benesh FS, Simmons M, Roberts R, et al. Cell-specific abnormalities of glutamate transporters in schizophrenia: sick astrocytes and compensating relay neurons? Mol Psychiatry. 2016;21:823–30.
O’Donovan SM, Hasselfeld K, Bauer D, Simmons M, Roussos P, Haroutunian V, et al. Glutamate transporter splice variant expression in an enriched pyramidal cell population in schizophrenia. Transl Psychiatry. 2015;5:e579.
Sodhi MS, Simmons M, McCullumsmith R, Haroutunian V, Meador-Woodruff JH. Glutamatergic gene expression is specifically reduced in thalamocortical projecting relay neurons in schizophrenia. Biol Psychiatry. 2011;70:646–54.
Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophr Res. 2013;147:32–8.
Kashihara K, Sato M, Fujiwara Y, Ogawa T, Fukuda K, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Yakubutsu Seishin Kodo = Japanese J Psychopharmacol. 1986;6:275–80.
Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502.
Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–94.
Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1.
Lu D, Insel PA. Hydrolysis of extracellular ATP by ectonucleoside triphosphate diphosphohydrolase (ENTPD) establishes the set point for fibrotic activity of cardiac fibroblasts. J Biol Chem. 2013;288:19040–9.
Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55.
Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–61.
Shan D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Expression of equilibrative nucleoside transporter type 1 protein in elderly patients with schizophrenia. Neuroreport. 2012a;23:224–7.
Wall MJ, Dale N. Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. J Physiol. 2013;591:3853–71.
Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev. 2001;21:105–28.
Brunstein MG, Silveira EM Jr., Chaves LS, Machado H, Schenkel O, Belmonte-de-Abreu P, et al. Increased serum adenosine deaminase activity in schizophrenic receiving antipsychotic treatment. Neurosci Lett. 2007;414:61–4.
Dutra GP, Ottoni GL, Lara DR, Bogo MR. Lower frequency of the low activity adenosine deaminase allelic variant (ADA1*2) in schizophrenic patients. Rev Bras De Psiquiatr. 2010;32:275–8.
Shan D, Yates S, Roberts RC, McCullumsmith RE. Update on the neurobiology of schizophrenia: a role for extracellular microdomains. Minerva Psichiatr. 2012b;53:233–49.
Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270.
Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, et al. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–50.
McCullumsmith RE, Hammond JH, Shan D, Meador-Woodruff JH. Postmortem brain: an underutilized substrate for studying severe mental illness. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2015;40:1307.
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci: Off J Soc Neurosci. 2014;34:11929–47.
Seibt KJ, Oliveira Rda L, Bogo MR, Senger MR, Bonan CD. Investigation into effects of antipsychotics on ectonucleotidase and adenosine deaminase in zebrafish brain. Fish Physiol Biochem. 2015;41:1383–92.
Seibt KJ, Oliveira Rda L, Rico EP, Dias RD, Bogo MR, Bonan CD. Antipsychotic drugs inhibit nucleotide hydrolysis in zebrafish (Danio rerio) brain membranes. Toxicol Vitr: Int J Publ Assoc BIBRA. 2009;23:78–82.
Acknowledgements
We thank Dr. Terry Kirley for his assistance and useful discussions on ENTPDs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Donovan, S.M., Sullivan, C., Koene, R. et al. Cell-subtype-specific changes in adenosine pathways in schizophrenia. Neuropsychopharmacol 43, 1667–1674 (2018). https://doi.org/10.1038/s41386-018-0028-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0028-6
This article is cited by
-
Glial cell deficits are a key feature of schizophrenia: implications for neuronal circuit maintenance and histological differentiation from classical neurodegeneration
Molecular Psychiatry (2025)
-
Neuronal alterations in AKT isotype expression in schizophrenia
Molecular Psychiatry (2025)
-
Exploring the Sensitivity of Peripheral ADA Levels Measurement in Establishing Psychosis Susceptibility
Journal of Molecular Neuroscience (2025)
-
Ancestral retrovirus envelope protein ERVWE1 upregulates circ_0001810, a potential biomarker for schizophrenia, and induces neuronal mitochondrial dysfunction via activating AK2
Cell & Bioscience (2024)
-
Impact of Vitamin D deficiency on immunological and metabolic responses in women with recurrent pregnancy loss: focus on VDBP/HLA-G1/CTLA-4/ENTPD1/adenosine-fetal-maternal conflict crosstalk
BMC Pregnancy and Childbirth (2024)