Abstract
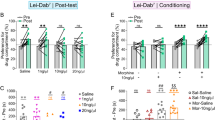
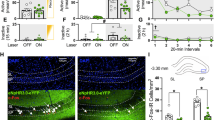
Contextual memory driven by abused drugs such as opiates has a central role in maintenance and relapse of drug-taking behaviors. Although dopamine (DA) signaling favors memory storage and retrieval via regulation of hippocampal–prefrontal connectivity, its role in modulating opiate-associated contextual memory is largely unknown. Here, we report roles of DA signaling within the hippocampal–prefrontal circuit for opiate-related memories. Combining-conditioned place preference (CPP) with molecular analyses, we investigated the DA D1 receptor (D1R) and extracellular signal-regulated kinase (ERK)-cAMP-response element binding protein (CREB) signaling, as well as DA D2 receptor (D2R) and protein kinase B (PKB or Akt)/glycogen synthase kinase 3 (GSK3) signaling in the ventral hippocampus (vHip) and medial prefrontal cortex (mPFC) during the formation of opiate-related associative memories. Morphine-CPP acquisition increased the activity of the D1R–ERK–CREB pathway in both the vHip and mPFC. Morphine-CPP reinstatement was associated with the D2R-mediated hyperactive GSK3 via Akt inhibition in the vHip and PFC. Furthermore, integrated D1R–ERK–CREB and D2R–Akt–GSK3 pathways in the vHip–mPFC circuit are required for the acquisition and retrieval of the morphine contextual memory, respectively. Moreover, blockage of D1R or D2R signaling could alleviate normal Hip-dependent spatial memory. These results suggest that D1R and D2R signaling are differentially involved in the acquisition and retrieval of morphine contextual memory, and DA signaling in the vHip–mPFC connection contributes to morphine-associated and normal memory, largely depending on opiate exposure states.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–22.
Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13.
Pirot S, Godbout R, Mantz J, Tassin JP, Glowinski J, Thierry AM. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–65.
McNamara CG, Dupret D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017;40:383–4.
Kutlu MG, Gould TJ. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem. 2016;23:515–33.
Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal–prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106.
Goto Y, Grace AA. Dopamine modulation of hippocampal–prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18:1407–14.
Esmaeili MH, Kermani M, Parvishan A, Haghparast A. Role of D1/D2 dopamine receptors in the CA1 region of the rat hippocampus in the rewarding effects of morphine administered into the ventral tegmental area. Behav Brain Res. 2012;231:111–5.
Assar N, Mahmoudi D, Farhoudian A, Farhadi MH, Fatahi Z, Haghparast A. D1- and D2-like dopamine receptors in the CA1 region of the hippocampus are involved in the acquisition and reinstatement of morphine-induced conditioned place preference. Behav Brain Res. 2016;312:394–404.
Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50.
Xing B, Li YC, Gao WJ. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016;1641:217–33.
Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73.
Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–47.
Kirschmann EK, Mauna JC, Willis CM, Foster RL, Chipman AM, Thiels E. Appetitive cue-evoked ERK signaling in the nucleus accumbens requires NMDA and D1 dopamine receptor activation and regulates CREB phosphorylation. Learn Mem. 2014;21:606–15.
Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology. 2010;35:955–66.
Miller JS, Barr JL, Harper LJ, Poole RL, Gould TJ, Unterwald EM. The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PLoS ONE. 2014;9:e88026.
Wang Y, Lai J, Cui H, Zhu Y, Zhao B, Wang W, et al. Inhibition of histone deacetylase in the basolateral amygdala facilitates morphine context-associated memory formation in rats. J Mol Neurosci 2015;55:269–278.
Banks PJ, Burroughs AC, Barker GR, Brown JT, Warburton EC, Bashir ZI. Disruption of hippocampal–prefrontal cortex activity by dopamine D2R-dependent LTD of NMDAR transmission. Proc Natl Acad Sci USA. 2015;112:11096–101.
Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex. 2010;20:393–403.
Wang L, Yuan LL. Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J Physiol. 2009;587:5139–47.
Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–5.
Hansen N, Manahan-Vaughan D. Dopamine D1/D5 receptors mediate informational saliency that promotes persistent hippocampal long-term plasticity. Cereb Cortex. 2014;24:845–58.
Puig MV, Antzoulatos EG, Miller EK. Prefrontal dopamine in associative learning and memory. Neuroscience. 2014;282:217–29.
Xing B, Kong H, Meng X, Wei SG, Xu M, Li SB. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169:1511–9.
Gangarossa G, Ceolin L, Paucard A, Lerner-Natoli M, Perroy J, Fagni L, et al. Repeated stimulation of dopamine D1-like receptor and hyperactivation of mTOR signaling lead to generalized seizures, altered dentate gyrus plasticity, and memory deficits. Hippocampus. 2014;24:1466–81.
Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84.
Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5.
Xing B, Guo J, Meng X, Wei SG, Li SB. The dopamine D1 but not D3 receptor plays a fundamental role in spatial working memory and BDNF expression in prefrontal cortex of mice. Behav Brain Res. 2012;235:36–41.
Li SX, Wang ZR, Li J, Peng ZG, Zhou W, Zhou M, et al. Inhibition of Period1 gene attenuates the morphine-induced ERK–CREB activation in frontal cortex, hippocampus, and striatum in mice. Am J Drug Alcohol Abus. 2008;34:673–82.
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–64.
Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003;13:123–35.
Nyberg L, Karalija N, Salami A, Andersson M, Wahlin A, Kaboovand N, et al. Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA. 2016;113:7918–23.
Takahashi H, Kato M, Takano H, Arakawa R, Okumura M, Otsuka T, et al. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci. 2008;28:12032–8.
Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM. Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology. 2014;231:3109–18.
Lin X, Wang Q, Ji J, Yu LC. Role of MEK-ERK pathway in morphine-induced conditioned place preference in ventral tegmental area of rats. J Neurosci Res. 2010;88:1595–604.
Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA. 2006;103:2932–7.
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–20.
Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85.
Funding
This work was supported by the National Science Foundation of China (81501636, 81401108), the Basic Science Research Programs of Shaanxi Province (2016JQ8003), and the Postdoctoral Science Foundation of China (2015M582673).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, H., Cui, J. et al. Opiate-associated contextual memory formation and retrieval are differentially modulated by dopamine D1 and D2 signaling in hippocampal–prefrontal connectivity. Neuropsychopharmacol 44, 334–343 (2019). https://doi.org/10.1038/s41386-018-0068-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0068-y
This article is cited by
-
Repeated Use of Morphine Induces Anxiety by Affecting a Proinflammatory Cytokine Signaling Pathway in the Prefrontal Cortex in Rats
Molecular Neurobiology (2023)
-
Anxiety and cognitive-related effects of Δ 9-tetrahydrocannabinol (THC) are differentially mediated through distinct GSK-3 vs. Akt-mTOR pathways in the nucleus accumbens of male rats
Psychopharmacology (2022)
-
Effects of heroin self-administration and forced withdrawal on the expression of genes related to the mTOR network in the basolateral complex of the amygdala of male Lewis rats
Psychopharmacology (2022)
-
Dopamine D1 receptor-mediated activation of the ERK signaling pathway is involved in the osteogenic differentiation of bone mesenchymal stem cells
Stem Cell Research & Therapy (2020)
-
Common neurocircuitry mediating drug and fear relapse in preclinical models
Psychopharmacology (2019)