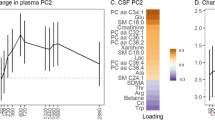
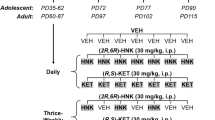
Abstract
(R,S)-Ketamine has rapid and sustained antidepressant effects in depressed patients. Although the metabolism of (R,S)-ketamine to (2 R,6 R)-hydroxynorketamine (HNK), a metabolite of (R)-ketamine, has been reported to be essential for its antidepressant effects, recent evidence suggests otherwise. The present study investigated the role of the metabolism of (R)-ketamine to (2 R,6 R)-HNK in the antidepressant actions of (R)-ketamine. Antidepressant effects were evaluated using the forced swimming test in the lipopolysaccharide (LPS)-induced inflammation model of mice and the tail suspension test in naive mice. To prevent the metabolism of (R)-ketamine to (2 R,6 R)-HNK, mice were pretreated with cytochrome P450 (CYP) inhibitors. The concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK in plasma, brain, and cerebrospinal fluid (CSF) samples were determined using enantioselective liquid chromatography-tandem mass spectrometry. The concentrations of (R)-norketamine and (2 R,6 R)-HNK in plasma, brain, and CSF samples after administration of (R)-norketamine (10 mg/kg) and (2 R,6 R)-HNK (10 mg/kg), respectively, were higher than those generated after administration of (R)-ketamine (10 mg/kg). Nonetheless, while (R)-ketamine attenuated, neither (R)-norketamine nor (2 R,6 R)-HNK significantly altered immobility times of LPS-treated mice. Treatment with CYP inhibitors prior to administration of (R)-ketamine increased the plasma levels of (R)-ketamine, while generation of (2 R,6 R)-HNK was almost completely blocked. (R)-Ketamine exerted the antidepressant effects at a lower dose in the presence of CYP inhibitors than in their absence, which is consistent with exposure levels of (R)-ketamine but not (2 R,6 R)-HNK. These results indicate that metabolism to (2 R,6 R)-HNK is not necessary for the antidepressant effects of (R)-ketamine and that unmetabolized (R)-ketamine itself may be responsible for its antidepressant actions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Hashimoto K. Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets. 2016;20:1389–92.
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72.
Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28.
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014a;116:137–41.
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361:9–16.
Adams JD Jr, Baillie TA, Trevor AJ, Castagnoli N Jr. Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom. 1981;8:527–38.
Zarate CA,Jr .Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–8.
Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–87.
Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. Int J Neuropsychopharmacol. 2018;21:84–8.
Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. R)-Ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry. 2017;82:e83–84.
Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur Arch Psychiatry Clin Neurosci. 2017;267:177–82.
Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014b;18 https://doi.org/10.1093/ijnp/pyu077.
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. 2017;39:134–44.
Villard V, Meunier J, Chevallier N, Maurice T. Pharmacological interaction with thesigma1 (σ1)-receptor in the acute behavioral effects of antidepressants. J Pharmacol Sci. 2011;115:279–92.
Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69.
Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–3.
Watanabe A, Mayumi K, Nishimura K, Osaki H. In vivo use of the CYP inhibitor 1-aminobenzotriazole to increase long-term exposure in mice. Biopharm Drug Dispos. 2016;37:373–8.
Balani SK, Li P, Nguyen J, Cardoza K, Zeng H, Mu DX, et al. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in guinea pigs and mice using serial sampling. Drug Metab Dispos. 2004;32:1092–5.
Balani SK, Zhu T, Yang TJ, Liu Z, He B, Lee FW. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in rats, dogs, and monkeys. Drug Metab Dispos. 2002;30:1059–62.
Molnari JC, Hassan HE, Moeller BM, Myers AL. Drug interaction study between bupropion and ticlopidine in male CF-1 mice. Biol Pharm Bull. 2011;34:447–51.
Peltoniemi MA, Saari TI, Hagelberg NM, Reponen P, Turpeinen M, Laine K, et al. Exposure to oral S-ketamine is unaffected by itraconazole but greatly increased by ticlopidine. Clin Pharmacol Ther. 2011;90:296–302.
Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC et al. Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry. 2018. https://doi.org/10.1016/j.biopsych.2017.10.020.
Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2018. https://doi.org/10.1038/mp.2017.239.
Acknowledgements
We thank Drs. Takashi Hashihayata, Dai Nozawa, and Masahiro Abe of Taisho Pharmaceutical Co., Ltd. for providing authentic standards for (R)-ketamine and (2 R,6 R)-HNK. We also thank Dr. Rodney W. Stevens of Taisho Pharmaceutical Co., Ltd. for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.Y. was supported by a Research Fellowship of the Japan Society for the Promotion of Science. K.H. is an inventor named on a filed patent application for “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. K.H. has received research support from Dainippon Sumitomo, Mochida, Otsuka, and Taisho. J.Y., H.T., H.K., A.M.Y., and S.C. are employees of Taisho Pharmaceutical Co., Ltd. Y.Q. declares no biomedical financial interests or potential conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yamaguchi, Ji., Toki, H., Qu, Y. et al. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacol 43, 1900–1907 (2018). https://doi.org/10.1038/s41386-018-0084-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0084-y
This article is cited by
-
The effects of low-dose ketamine and (2R,6R)-Hydroxynorketamine on affective behaviors associated with protracted oxycodone withdrawal
Psychopharmacology (2025)
-
Ketamine and its enantiomers for depression: a bibliometric analysis from 2000 to 2023
European Archives of Psychiatry and Clinical Neuroscience (2025)
-
Comments to behavioral tests for antidepressant-like actions of (2R,6R)–hydroxynorketamine by Bonaventura et al.
Molecular Psychiatry (2024)
-
The role of neurotrophic factors in novel, rapid psychiatric treatments
Neuropsychopharmacology (2024)
-
Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor
Molecular Psychiatry (2022)