Abstract
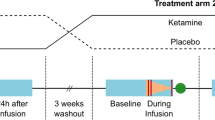
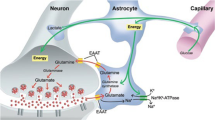
The ability of ketamine administration to activate prefrontal glutamate neurotransmission is thought to be a key mechanism contributing to its transient psychotomimetic effects and its delayed and sustained antidepressant effects. Rodent studies employing carbon-13 magnetic resonance spectroscopy (13C MRS) methods have shown ketamine and other N-methyl-D-aspartate (NMDA) receptor antagonists to transiently increase measures reflecting glutamate–glutamine cycling and glutamate neurotransmission in the frontal cortex. However, there are not yet direct measures of glutamate neurotransmission in vivo in humans to support these hypotheses. The current first-level pilot study employed a novel prefrontal 13C MRS approach similar to that used in the rodent studies for direct measurement of ketamine effects on glutamate–glutamine cycling. Twenty-one participants (14 healthy and 7 depressed) completed two 13C MRS scans during infusion of normal saline or subanesthetic doses of ketamine. Compared to placebo, ketamine increased prefrontal glutamate–glutamine cycling, as indicated by a 13% increase in 13C glutamine enrichment (t = 2.4, p = 0.02). We found no evidence of ketamine effects on oxidative energy production, as reflected by 13C glutamate enrichment. During ketamine infusion, the ratio of 13C glutamate/glutamine enrichments, a putative measure of neurotransmission strength, was correlated with the Clinician-Administered Dissociative States Scale (r = −0.54, p = 0.048). These findings provide the most direct evidence in humans to date that ketamine increases glutamate release in the prefrontal cortex, a mechanism previously linked to schizophrenia pathophysiology and implicated in the induction of rapid antidepressant effects.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214.
Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–86.
Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38:958–66.
Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38:942–9.
Krystal JH, Berman RM, Skolnick P, Paul IA, Sanacora G. Glutamate and the pathophysiology and treatment of depression. Biol Psychiatry. 1999;45:1S–47S.
de Graaf RA, Rothman DL, Behar KL. State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide. NMR Biomed. 2011;24:958–72.
Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, et al. Glutamate metabolism in major depressive disorder. Am J Psychiatry. 2014;171:1320–7.
Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–57.
Li S, Zhang Y, Wang S, Araneta MF, Johnson CS, Xiang Y, et al. 13C MRS of occipital and frontal lobes at 3 T using a volume coil for stochastic proton decoupling. NMR Biomed. 2010;23:977–85.
Li S, Zhang Y, Wang S, Yang J, Ferraris Araneta M, Farris A, et al. In vivo 13C magnetic resonance spectroscopy of human brain on a clinical 3 T scanner using [2-13C]glucose infusion and low-power stochastic decoupling. Magn Reson Med. 2009;62:565–73.
Sailasuta N, Robertson LW, Harris KC, Gropman AL, Allen PS, Ross BD. Clinical NOE 13C MRS for neuropsychiatric disorders of the frontal lobe. J Magn Reson. 2008;195:219–25.
Hare B, Ghosal S, Duman R. Rapid acting antidepressants in chronic stress models: molecular and cellular mechanisms. Chronic Stress. 2017;1:1–16.
Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018. https://doi.org/10.1016/j.pharmthera.2018.05.010.
Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. 1H-[13C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–5.
Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–6.
Hyder F, Rothman DL, Bennett MR. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci USA. 2013;110:3549–54.
Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–95.
Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–64.
Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl). 2005;179:303–9.
Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–93.
Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–6.
Chen H, De Feyter HM, Brown PB, Rothman DL, Cai S, de Graaf RA. Comparison of direct (13)C and indirect (1)H-[(13)C] MR detection methods for the study of dynamic metabolic turnover in the human brain. J Magn Reson. 2017;283:33–44.
D’Souza DC, Singh N, Elander J, Carbuto M, Pittman B, de Haes JU, et al. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37:1036–46.
Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D’Souza DC, et al. Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry. 2012;71:969–77.
Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–12.
Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–5.
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–11.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–204.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–22.
Javitt DC, Carter CS, Krystal JH, Kantrowitz JT, Girgis RR, Kegeles LS, et al. Utility of imaging-based biomarkers for glutamate-targeted drug development in psychotic disorders: a randomized clinical trial. JAMA Psychiatry. 2018;75:11–9.
Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol. 1997;7:25–38.
Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol. 1997;7:9–24.
Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, et al. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77:569–80.
Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 2012;109:16720–5.
Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:566–74.
Davis MT, Holmes SE, Pietrzak RH, Esterlis I. Neurobiology of chronic stress-related psychiatric disorders: evidence from molecular imaging studies. Chronic Stress. 2017;1. https://doi.org/10.1177/2470547017710916.
DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, et al. In vivo ketamine-induced changes in [(11)C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol Psychiatry. 2015;77:266–75.
Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [(11)C]ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824–32.
Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry. 2016;21:320–7.
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–6.
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–5.
Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26:733–7.
Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28:2020–8.
Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–82.
De Feyter HM, Herzog RI, Steensma BR, Klomp DWJ, Brown PB, Mason GF, et al. Selective proton-observed, carbon-edited (selPOCE) MRS method for measurement of glutamate and glutamine (13) C-labeling in the human frontal cortex. Magn Reson Med. 2018;80:11–20.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7.
Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33:689–97.
Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry. 2018;84:e3–6.
Schwarcz R, Wu H-Q, Zanos P, Gould T. Endogenous kynurenic acid mediates ketamine- and (2R,6R)-hydroxynorketamine-induced increases in extracellular glutamate and antidepressant actions. Neuropsychopharmacology. 2017;43:S111.
Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–9.
Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–94.
Fukumoto K, Kato T, Li X-Y, Duman R. Role of BDNF/mTORC1 signaling in the long-lasting antidepressant effects of (2R,6R)-hydroxynorketamine. Neuropsychopharmacology. 2017;43:S111.
Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83:29–37.
Zanos P, Gould TD. Intracellular signaling pathways involved in (S)- and (R)-ketamine antidepressant actions. Biol Psychiatry. 2018;83:2–4.
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42:1210–9.
Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, et al. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp. 2016;37:3214–23.
Scheinost D, Holmes SE, DellaGioia N, Schleifer C, Matuskey D, Abdallah CG, et al. Multimodal investigation of network level effects using intrinsic functional connectivity, anatomical covariance, and structure-to-function correlations in unmedicated major depressive disorder. Neuropsychopharmacology. 2018;43:1119–27.
Acknowledgments
The authors would like to thank the individuals who participated in this study for their invaluable contribution. Funding and research support were provided by NIMH (K23MH101498), NIAAA (R01AA021984), the VA National Center for PTSD, Brain & Behavior Foundation (NARSAD), Patterson Trust Award, American Psychiatric Foundation, Clinical Neuroscience Research Unit (CNRU) at Connecticut Mental Health Center, and Yale Center for Clinical Investigation (YCCI UL1 RR024139), an NIH Clinical and Translational Science Award (CTSA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors, the Department of Veterans Affairs, NIH, or the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CGA has served as a consultant and/or on advisory boards for Genentech and Janssen, and editor of “Chronic Stress” for Sage Publications, Inc. JHK is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol–Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries. JHK is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer. JHK is a stockholder in Biohaven Pharmaceuticals, holds stock options in Mnemosyne Pharmaceuticals, Inc., holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued September 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on March 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on June 17, 2014). GS reports personal consulting fees from Alkermes, Allergan, Biohaven Pharmaceuticals, Eli Lilly and Co., Genentech, Intra-Cellular Therapies, Janssen Pharmaceuticals, Lundbeck Research USA, Merck & Co., Naurex, Navitor Pharmaceuticals, Noven Pharmaceuticals, Teva Pharmaceuticals Industries, Taisho Pharmaceutical Co., Takeda Pharmaceutical Co., Sage Pharmaceuticals, Inc., Sevier, Valeant Pharmaceuticals, and Vistagen Therapeutics, Inc. GS has grants and research contracts from Eli Lilly and Co., Janssen Pharmaceuticals, Merck & Co., and Sevier; and support from Sanofi-Aventis, in the form of free medication for an NIH sponsored study over the last 36 months. In addition, Dr. GS is a stockholder and holds stock options in Biohaven Pharmaceuticals; and has a patent for Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued July 15, 2014) with royalties paid from Biohaven Pharmaceuticals. GFM is a consultant for Sumitomo Dainippon Pharma Co. Ltd. and UCB Pharma SA, and serves on the Scientific Advisory Board of Elucidata, Inc. All other authors report no competing interests.
Ethics approval
All study procedures were approved by an institutional review board (ClinicalTrials.gov NCT02037035).
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Abdallah, C.G., De Feyter, H.M., Averill, L.A. et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacol 43, 2154–2160 (2018). https://doi.org/10.1038/s41386-018-0136-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0136-3
This article is cited by
-
Can dextromethorphan-bupropion reduce mental pain in depressed individuals? A hypothesis-generating overview perspective
Annals of General Psychiatry (2025)
-
Activation of glutamine synthetase (GS) as a new strategy for the treatment of major depressive disorder and other GS-related diseases
Acta Pharmacologica Sinica (2025)
-
The therapeutic potential of psilocybin beyond psychedelia through shared mechanisms with ketamine
Molecular Psychiatry (2025)
-
Effect of naltrexone pretreatment on ketamine-induced glutamatergic activity and symptoms of depression: a randomized crossover study
Nature Medicine (2025)
-
Challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression
Neuropsychopharmacology (2025)