Abstract
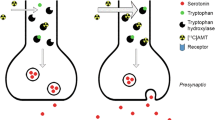
In neuropharmacology, the recent concept of 'biased agonism' denotes the capacity of certain agonists to target-specific intracellular pathways of a given receptor in specific brain areas. In the context of serotonin pharmacotherapy, 5-HT1A receptor-biased agonists could be of great interest in several neuropsychiatric disorders. The aim of this study was to determine whether biased agonists could be differentiated in terms of regional targeting by use of simultaneous functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) brain imaging. We compared two 5-HT1A-biased agonists, NLX-112 and NLX-101, injected at three different doses in anaesthetised cats (n = 4). PET imaging was acquired for 90 min after bolus administration followed by constant infusion of the 5-HT1A radiotracer, [18F]MPPF. Drug occupancy was evaluated after injection at 50 min and BOLD fMRI was simultaneously acquired to evaluate subsequent brain activation patterns. 5-HT1A receptor occupancy was found to be dose-dependent for both agonists, but differed in magnitude and spatial distribution at equal doses with distinct BOLD patterns. Functional connectivity, as measured by BOLD signal temporal correlations between regions, was also differently modified by NLX-112 or NLX-101. Voxel-based correlation analyses between PET and fMRI suggested that NLX-112 stimulates both 5-HT1A autoreceptors and post-synaptic receptors, whereas NLX-101 preferentially stimulates post-synaptic cortical receptors. In cingulate cortex, the agonists induced opposite BOLD signal changes in response to receptor occupancy. These data constitute the first simultaneous exploration of 5-HT1A occupancy and its consequences in terms of brain activation, and demonstrates differential signalling by two 5-HT1A-biased agonists. Combined PET/fMRI represents a powerful tool in neuropharmacology, and opens new ways to address the concept of biased agonism by translational approaches.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Luttrell LM, Maudsley S, Bohn LM. Fulfilling the promise of “biased” G protein-coupled receptor agonism. Mol Pharmacol. 2015;88:579–88.
Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol. 2006;70:1013–21.
Newman-Tancredi A. Biased agonism at serotonin 5-HT1A receptors: preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry. 2011;1:149–64.
Abdala AP, Bissonnette JM, Newman-Tancredi A. Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: therapeutic perspectives for 5-HT1A agonists. Front Physiol. 2014;5:205.
Iderberg H, McCreary AC, Varney MA, Kleven MS, Koek W, Bardin L, et al. NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: behavioral and neurochemical profile in rat. Exp Neurol. 2015;271:335–50.
Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009;156:338–53.
Newman-Tancredi A, Martel JC, Cosi C, Heusler P, Lestienne F, Varney MA, et al. Distinctive in vitro signal transduction profile of NLX-112, a potent and efficacious serotonin 5-HT1A receptor agonist. J Pharm Pharmacol. 2017;69:1178–90.
Llado-Pelfort L, Assie MB, Newman-Tancredi A, Artigas F, Celada P. In vivo electrophysiological and neurochemical effects of the selective 5-HT1A receptor agonist, F13640, at pre- and postsynaptic 5-HT1A receptors in the rat. Psychopharmacology. 2012;221:261–72.
Buritova J, Berrichon G, Cathala C, Colpaert F, Cussac D. Region-specific changes in 5-HT1A agonist-induced extracellular signal-regulated kinases 1/2 phosphorylation in rat brain: a quantitative ELISA study. Neuropharmacology. 2009;56:350–61.
Becker G, Bolbos R, Costes N, Redouté J, Newman-Tancredi A, Zimmer L. Selective serotonin 5-HT1A receptor biased agonists elicit distinct brain activation patterns: a pharmacoMRI study. Sci Rep. 2016;6:26633.
Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27:657–60.
Aznavour N, Rbah L, Léger L, Buda C, Sastre JP, Imhof A, et al. A comparison of in vivo and in vitro neuroimaging of 5-HT 1A receptor binding sites in the cat brain. J Chem Neuroanat. 2006a;31:226–32.
Aznavour N, Rbah L, Riad M, Reilhac A, Costes N, Descarries L, et al. A PET imaging study of 5-HT(1A) receptors in cat brain after acute and chronic fluoxetine treatment. NeuroImage. 2006b;33:834–42.
Lancelot S, Costes N, Lemoine L, Zimmer L. Development and evaluation of a digital atlas for PET neuroimaging in domestic cat (Felis catus). Eur J Nucl Med Mol Imaging. 2010;37:S387–7.
Casanova R, Ryali S, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. NeuroImage. 2007;34:137–43.
Ginovart N, Hassoun W, Le Bars D, Weissmann D, Leviel V. In vivo characterization of p-[(18)F]MPPF, a fluoro analog of WAY-100635 for visualization of 5-HT(1a) receptors. Synapse. 2000;35:192–200.
Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. NeuroImage. 2012;62:1072–85.
Zimmer L, Luxen A. PET radiotracers for molecular imaging in the brain: past, present and future. NeuroImage. 2012;61:363–70.
Hansen HD, Mandeville JB, Sander CY, Hooker JM, Catana C, Rosen BR, et al. Functional characterization of 5-HT1B receptor drugs in non-human primates using simultaneous PET-MR. J Neurosci. 2017;37:10671–8.
Mandeville JB, Liu CH, Vanduffel W, Marota JJ, Jenkins BG. Data collection and analysis strategies for phMRI. Neuropharmacology. 2014;84:65–78.
Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci USA. 2013;110:11169–74.
Sander CY, Hooker JM, Catana C, Rosen BR, Mandeville JB. Imaging agonist-induced D2/D3 receptor desensitization and internalization in vivo with PET/fMRI. Neuropsychopharmacology. 2016;41:1427–36.
Wey HY, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, et al. Simultaneous fMRI-PET of the opioidergic pain system in human brain. NeuroImage. 2014;102:275–82.
Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung SC, Tamir H, et al. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl- 11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging. 2007;34:1050–60.
Shrestha SS, Liow JS, Lu S, Jenko K, Gladding RL, Svenningsson P, et al. 11)C-CUMI-101, a PET radioligand, behaves as a serotonin 1A receptor antagonist and also binds to α(1) adrenoceptors in brain. J Nucl Med. 2014;55:141–6.
Hirani E, Opacka-Juffry J, Gunn R, Khan I, Sharp T, Hume S. Pindolol occupancy of 5-HT(1A) receptors measured in vivo using small animal positron emission tomography with carbon-11 labeled WAY 100635. Synapse. 2000;36:330–41.
Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, et al. Differential occupancy of somatodendritic and postsynaptic 5HT(1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24:209–29.
Maurel JL, Autin JM, Funes P, Newman-Tancredi A, Colpaert F, Vacher B. High-efficacy 5-HT1A agonists for antidepressant treatment: a renewed opportunity. J Med Chem. 2007;50:5024–33.
Colpaert FC, Tarayre JP, Koek W, Pauwels JP, Bardin L, Xu XJ, et al. Large-amplitude 5-HT1A receptor activation: a new mechanism of profound, central analgesia. Neuropharmacology. 2002;43:945–58.
McCreary AC, Varney MA, Newman-Tancredi A. The novel 5-HT1A receptor agonist, NLX-112 reduces l-DOPA-induced abnormal involuntary movements in rat: a chronic administration study with microdialysis measurements. Neuropharmacology. 2016;105:651–60.
Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse. 2006;59:18–26.
Zimmer L, Mauger G, Le Bars D, Bonmarchand G, Luxen A, Pujol JF. Effect of endogenous serotonin on the binding of the 5-hT1A PET ligand 18F-MPPF in the rat hippocampus: kinetic beta measurements combined with microdialysis. J Neurochem. 2002;80:278–86.
Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. NeuroImage. 2012;62:1040–50.
Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–9.
Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010;22:1406–12.
Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology. 2005;180:687–704.
Andrade R, Huereca D, Lyons JG, Andrade EM, McGregor KM. 5-HT1A receptor-mediated autoinhibition and the control of serotonergic cell firing. ACS Chem Neurosci. 2015;6:1110–5.
McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, et al. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology. 2005;180:680–6.
Preece MA, Taylor MJ, Raley J, Blamire A, Sharp T, Sibson NR. Evidence that increased 5-HT release evokes region-specific effects on blood-oxygenation level-dependent functional magnetic resonance imaging responses in the rat brain. Neuroscience. 2009;159:751–9.
Razoux F, Baltes C, Mueggler T, Seuwen A, Russig H, Mansuy I, et al. Functional MRI to assess alterations of functional networks in response to pharmacological or genetic manipulations of the serotonergic system in mice. NeuroImage. 2013;74:326–36.
Depoortere R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol. 2010;20:641–54.
Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, et al. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46:1980–9.
Aznavour N, Zimmer L. [18F]MPPF as a tool for the in vivo imaging of 5-HT1A receptors in animal and human brain. Neuropharmacology. 2007;52:695–707.
Charnay Y, Leger L, Vallet PG, Greggio B, Hof PR, Cespuglio R, et al. Mapping of 5-HT1a receptor binding sites in the feline brain: a quantitative autoradiographic study using [3H]8-OH-DPAT. Biog Amines. 1997;13:217–32.
Massey CA, Iceman KE, Johansen SL, Wu Y, Harris MB, Richerson GB. Isoflurane abolishes spontaneous firing of serotonin neurons and masks their pH/CO(2) chemosensitivity. J Neurophysiol. 2015;113:2879–88.
Seeman P, Kapur S. Anesthetics inhibit high-affinity states of dopamine D2 and other G-linked receptors. Synapse. 2003;50:35–40.
Haensel JX, Spain A, Martin C. A systematic review of physiological methods in rodent pharmacological MRI studies. Psychopharmacology. 2015;232:489–99.
Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–16.
Acknowledgements
We thank Thomas Troalen (Siemens Healthiness France) for assistance with the PET/MR camera, Thibault Iecker and Christian Tourvielle for the production of [18F]MPPF and Véronique Gualda for the zootechnical assistance.
Funding:
This work was supported by the Fondation NEURODIS, the IHU CESAME and the French national programme ‘Investissement d’Avenir’ Programmes (LILI – Lyon Integrated Life Imaging: hybrid MR-PET ANR-11-EQPX-0026), and performed within the framework of the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon, within the programme 'Investissements d’Avenir' (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AN-T is an employee and stockholder of Neurolixis. The remaining authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vidal, B., Fieux, S., Redouté, J. et al. In vivo biased agonism at 5-HT1A receptors: characterisation by simultaneous PET/MR imaging. Neuropsychopharmacol 43, 2310–2319 (2018). https://doi.org/10.1038/s41386-018-0145-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0145-2
This article is cited by
-
Towards in vivo imaging of functionally active 5-HT1A receptors in schizophrenia: concepts and challenges
Translational Psychiatry (2021)