Abstract
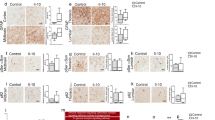
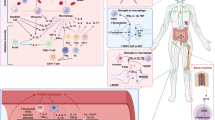
In humans, depression is often associated with low-grade inflammation, activation of the tryptophan/kynurenine pathway, and mild lymphopenia. Preclinical research confirms that inflammation induces depression-like behavior through activation of the tryptophan/kynurenine pathway. However, the mechanisms governing recovery from depression are unknown. Understanding the pathways leading to resolution of depression will likely lead to identification of novel targets for treatment. We investigated the contribution of T lymphocytes to the resolution of lipopolysaccharide-induced depression-like behavior. Duration of depression-like behavior was markedly prolonged in mice without mature T or B lymphocytes (Rag1−/− mice). This prolonged depression-like behavior was associated with persistent upregulation of the tryptophan-metabolizing enzyme indoleamine-2,3-dioxygenase (Ido)1 in the prefrontal cortex (PFC). Reconstitution of Rag1−/− mice with T lymphocytes normalized resolution of depression-like behavior and expression of Ido1 in the PFC. During resolution of inflammation-induced depression-like behavior, T lymphocytes accumulated in the meninges and were required for induction of interleukin (IL)-10 in the meninges and the PFC. Inhibition of IL-10 signaling by nasal administration of neutralizing anti–IL-10 antibody to WT mice led to persistent upregulation of Ido1 in the PFC and prolonged depression-like behavior. Conversely, nasal administration of recombinant IL-10 in Rag1−/− mice normalized Ido1 expression and resolution of depression-like behavior. In conclusion, the present data show for the first time that resolution of inflammation-induced depression is an active process requiring T lymphocytes acting via an IL-10–dependent pathway to decrease Ido1 expression in the brain. We propose that targeting the T lymphocyte/IL-10 resolution pathway could represent a novel approach to promote recovery from major depressive disorder.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
The burden of depression. Nature. 2014;515:163.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57.
Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709.
Grosse L, Hoogenboezem T, Ambree O, Bellingrath S, Jorgens S, de Wit HJ, et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun. 2016;54:38–44.
Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1–8.
Snijders G, Schiweck C, Mesman E, Grosse L, De Wit H, Nolen WA, et al. A dynamic course of T cell defects in individuals at risk for mood disorders. Brain Behav Immun. 2016;58:11–7.
Toben C, Baune BT. An act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J Neuroimmune Pharmacol. 2015;10:595–609.
Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–80.
Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370.
Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73.
Georgin-Lavialle S, Moura DS, Salvador A, Chauvet-Gelinier JC, Launay JM, Damaj G, et al. Mast cells’ involvement in inflammation pathways linked to depression: evidence in mastocytosis. Mol Psychiatry. 2016;21:1511–6.
Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, et al. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015;227:206–12.
Savitz J. Role of Kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci. 2017;31:249–67.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012a;122:2940–54.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Remus JL, Dantzer R. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int J Neuropsychopharmacol. 2016;19:pyw028.
Maciel IS, Silva RB, Morrone FB, Calixto JB, Campos MM. Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS ONE. 2013;8:e77227.
O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009a;29:4200–9.
Zhou W, Dantzer R, Budac DP, Walker AK, Mao-Ying QL, Lee AW, et al. Peripheral indoleamine 2,3-dioxygenase 1 is required for comorbid depression-like behavior but does not contribute to neuropathic pain in mice. Brain Behav Immun. 2015;46:147–53.
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009b;14:511–22.
Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–87.
Ellwardt E, Walsh JT, Kipnis J, Zipp F. Understanding the role of T cells in CNS homeostasis. Trends Immunol. 2016;37:154–65.
Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–9.
Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–80.
Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–8.
Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–8.
Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4 + CD25 + cells. J Neurobiol. 2006;66:552–63.
Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55.
Raposo C, Graubardt N, Cohen M, Eitan C, London A, Berkutzki T, et al. CNS repair requires both effector and regulatory T cells with distinct temporal and spatial profiles. J Neurosci. 2014;34:10141–55.
Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, et al. MHCII-independent CD4 + T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699–714.
Xin J, Wainwright DA, Mesnard NA, Serpe CJ, Sanders VM, Jones KJ. IL-10 within the CNS is necessary for CD4 + T cells to mediate neuroprotection. Brain Behav Immun. 2011;25:820–9.
Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77.
Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66:80–101.
Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, et al. CD8 + T Cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36:11074–83.
Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, et al. IL4-10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci. 2016;36:7353–63.
van Vulpen LFD, Popov-Celeketic J, van Meegeren MER, Coeleveld K, van Laar JM, Hack CE, et al. A fusion protein of interleukin-4 and interleukin-10 protects against blood-induced cartilage damage in vitro and in vivo. J Thromb Haemost. 2017;15:1788–98.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–22.
Mayo L, Cunha AP, Madi A, Beynon V, Yang Z, Alvarez JI, et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain. 2016;139(Pt 7):1939–57.
Rosso P, Moreno S, Fracassi A, Rocco ML, Aloe L. Nerve growth factor and autophagy: effect of nasal anti-NGF-antibodies administration on Ambra1 and Beclin-1 expression in rat brain. Growth Factors. 2015;33:401–9.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Jo WK, Zhang Y, Emrich HM, Dietrich DE. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Front Cell Neurosci. 2015;9:268.
Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, et al. CD4+ CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS ONE. 2012b;7:e42054.
Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: prevalence and measurement. Pain Pract. 2009;9:173–80.
Bodeman CE, Dzierlenga AL, Tally CM, Mulligan RM, Lake AD, Cherrington NJ, et al. Differential regulation of hepatic organic cation transporter 1, organic anion-transporting polypeptide 1a4, bile-salt export pump, and multidrug resistance-associated protein 2 transporter expression in lymphocyte-deficient mice associates with interleukin-6 production. J Pharmacol Exp Ther. 2013;347:136–44.
Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, et al. Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2(-/-) mice. Behav Brain Res. 2015;278:74–82.
Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, et al. ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 2013;4:e710.
Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflamm. 2016;13:297.
Wu Z, Tokuda Y, Zhang XW, Nakanishi H. Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiol Dis. 2008;32:543–51.
Wu Z, Zhang J, Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 2005;167:90–8.
Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42:46–61.
Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, et al. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain. 2014;15:496–506.
Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11.
Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, et al. Replicable and coupled changes in innate and adaptive immune gene expression in two case-control studies of blood microarrays in major depressive disorder. Biol Psychiatry. 2017;83:70–80.
Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, et al. Altered expression of CD4( + )CD25( + ) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord. 2010;124:68–75.
Holtzman S, Abbey SE, Chan C, Bargman JM, Stewart DE. A genetic predisposition to produce low levels of IL-10 is related to depressive symptoms: a pilot study of patients with end stage renal disease. Psychosomatics. 2012;53:155–61.
Acknowledgements
We thank Dr. Eric C. Hack (UMC Utrecht, Netherlands) for providing the recombinant IL-10 fusion protein. We thank Bryan LaVergne for technical assistance. We thank Drs. Jonathan Kipnis and Antoine Louveau (University of Virginia at Charlottesville) for sharing their expertise on harvesting the meninges. The authors acknowledge Jeanie F. Woodruff, BS, ELS, for editorial support. This work was supported by NIH grant R01 NS073939 (AK, RD, and CJH), a Cyrus Scholar Award (GL) and the American Pain Society Future Leader in Pain Research grant (GL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RD has received honorarium from Danone Nutricia Research France. The other authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laumet, G., Edralin, J.D., Chiang, A.CA. et al. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacol 43, 2597–2605 (2018). https://doi.org/10.1038/s41386-018-0154-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0154-1
This article is cited by
-
The immunological perspective of major depressive disorder: unveiling the interactions between central and peripheral immune mechanisms
Journal of Neuroinflammation (2025)
-
Investigating the Potential Mechanisms and Therapeutic Targets of Inflammatory Cytokines in Post-stroke Depression
Molecular Neurobiology (2024)
-
Intranasal Administration of Resolvin E1 Produces Antidepressant-Like Effects via BDNF/VEGF-mTORC1 Signaling in the Medial Prefrontal Cortex
Neurotherapeutics (2023)
-
Uliginosin B, a Natural Phloroglucinol, Increases Hippocampal GSH, MCP-1 and IL-10 Levels in Mice
Revista Brasileira de Farmacognosia (2023)
-
A novel 4 immune-related genes as diagnostic markers and correlated with immune infiltrates in major depressive disorder
BMC Immunology (2022)