Abstract
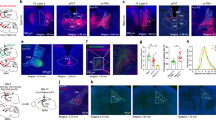
Acute exposure to a salient stressor, such as in post-traumatic stress disorder, can have lasting impacts upon an individual and society. To study stress in rodents, some naturalistic methods have included acute exposure to a predator odor, such as the synthetic fox odor 2,4,5, trimethyl-3-thiazoline (TMT). These experiments explore the stress-related behaviors and cortical activity induced by TMT exposure in adult male C57BL/6J mice and the influence of the stress neuropeptide corticotropin-releasing factor (CRF) on these responses. Compared to H2O and a novel odorant, vanilla, mice exposed to TMT in the home cage showed increased avoidance and defensive burying indicative of evident stress responses. Consistent with stress-induced activation of the medial prefrontal cortex (mPFC), we found that the prelimbic (PL) and infralimbic (IL) subregions of the mPFC had elevated c-Fos immunolabeling after TMT and vanilla compared to H2O. Slice physiology recordings were performed in layers 2/3 and 5 of the PL and IL, following TMT, vanilla, or H2O exposure. In TMT mice, but not vanilla or H2O mice, PL layers 2/3 showed heightened spontaneous excitatory post-synaptic currents and synaptic drive, suggesting TMT enhanced excitatory transmission. Synaptic drive in PL was increased in both TMT and H2O mice following bath application of 300 nM CRF, but only H2O mice increased excitatory currents with 100 nM CRF, suggesting dose-effect curve shifts in TMT mice. Further, systemic pretreatment with the CRF-R1 antagonist CP154526 and bath application with the CRF-R1 antagonist NBI27914 reduced excitatory transmission in TMT mice, but not H2O mice. CP154526 also reduced stress-reactive behaviors induced by TMT. Taken together, these findings suggest that exposure to TMT leads to CRF-R1 driven changes in behavior and changes in synaptic function in layer 2/3 neurons in the PL, which are consistent with previous findings that CRF-R1 in the mPFC plays an important role in predator odor-related behaviors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
22 January 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41386-024-01804-7
References
Yehuda R. Post-traumatic stress disorder. New Engl J Med. 2002;346:108–14.
Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–7.
Fendt M, Endres T. 2, 3, 5-Trimethyl-3-thiazoline (TMT), a component of fox odor–just repugnant or really fear-inducing? Neurosci Biobehav Rev. 2008;32:1259–66.
Rosen JB, Asok A, Chakraborty T. The smell of fear: innate threat of 2, 5-dihydro-2, 4, 5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci. 2015;9:292.
Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–51.
Janitzky K, Kröber A, Schwegler H. TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress. Brain Res. 2015;1599:1–8.
Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–508.
Schreiber AL, Lu YL, Baynes BB, Richardson HN, Gilpin NW. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology. 2017;113:323–30.
Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents Comparison with diazepam and buspirone. Psychopharmacology. 1998;138:55–66.
Keller C, Bruelisauer A, Lemaire M, Enz A. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002;30:173–6.
Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates, compact. The coronal plates and diagrams. Amsterdam: Elsevier Academic Press. 2008.
Pittaras EC, Faure A, Leray X, Moraitopoulou E, Cressant A, Rabat AA, et al. Neuronal nicotinic receptors are crucial for tuning of E/I balance in prelimbic cortex and for decision-making processes. Frontiers in Psychiatry. 2016;7:171.
Stewart CV, Plenz D. Inverted-U profile of dopamine–NMDA-mediated spontaneous avalanche recurrence in superficial layers of rat prefrontal cortex. J Neurosci. 2006;26:8148–59.
Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, et al. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545.
Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1 dependent process. J Neurosci. 2008;28:13856–65.
Pérez-Gómez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger SD, et al. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol. 2015;25:1340–6.
Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, et al. Mapping sub-second structure in mouse behavior. Neuron. 2015;88:1121–35.
De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–61.
Pinel JP, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978;92:708.
Silverman AP. Rodents’ defence against cigarette smoke. Anim Behav. 1978;26:1279–81.
Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci. 2002;116:160.
Yang H, Yang J, Xi W, Hao S, Luo B, He X, et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci. 2016;19:283.
Yang M, Farrokhi C, Vasconcellos A, Blanchard RJ, Blanchard DC. Central infusion of ovine CRF (oCRF) potentiates defensive behaviors in CD-1 mice in the Mouse Defense Test Battery (MDTB). Behav Brain Res. 2006;171:1–8.
Diamant M, Croiset G, de Wied D. The effect of corticotropin-releasing factor (CRF) on autonomic and behavioral responses during shock-prod burying test in rats. Peptides. 1992;13:1149–58.
Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, et al. Prefrontal cortex corticotropin-releasing factor receptor 1 conveys acute stress-induced executive dysfunction. Biol Psychiatry. 2016;80:743–53.
Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD‐1 mice. Eur J Neurosci. 2004;20:2415–29.
Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2, 5-dihydro-2, 4, 5-trimethylthiazoline (TMT). Behav Brain Res. 2013;248:85–93.
Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–50.
Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–94.
Miguel TT, Gomes KS, Nunes-de-Souza RL. Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: role of protein kinase A (PKA). Horm Behav. 2014;66:247–56.
Hwa LS, Shimamoto A, Kayyali T, Norman KJ, Valentino RJ, DeBold JF, et al. Dissociation of μ‐opioid receptor and CRF‐R1 antagonist effects on escalated ethanol consumption and mPFC serotonin in C57BL/6J mice. Addict Biol. 2016;21:111–24.
Smith DG, Davis RJ, Gehlert DR, Nomikos GG. Exposure to predator odor stress increases efflux of frontal cortex acetylcholine and monoamines in mice: comparisons with immobilization stress and reversal by chlordiazepoxide. Brain Res. 2006;1114:24–30.
Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Dev Neurobiol. 2001;49:245–53.
Radley JJ, Sisti HM, Hao J, Rocher A, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6.
Yang XD, Liao XM, Uribe-Mariño A, Liu R, Xie XM, Jia J, et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF 1. Neuropsychopharmacology. 2015;40:1203.
Eberly LB, Dudley CA, Moss RL. Iontophoretic mapping of corticotropin-releasing factor (CRF) sensitive neurons in the rat forebrain. Peptides. 1983;4:837–41.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61.
Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–73.
Musazzi L, Tornese P, Sala N, Popoli M. Acute stress is not acute: sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol Psychiatry. 2017;22:1226.
Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566.
Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433.
Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, & Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci. 2009;106:14075–9.
Caruso MJ, Crowley NA, Reiss DE, Caulfield JI, Luscher B, Cavigelli SA, et al. Adolescent social stress increases anxiety-like behavior and alters synaptic transmission, without influencing nicotine responses, in a sex-dependent manner. Neuroscience. 2018;373:182–98.
Urban KR, Valentino RJ. Age-and sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cereb Cortex. 2017;27:244–53.
Liu RJ, Aghajanian GK. Stress blunts serotonin-and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci. 2008;105:359–64.
Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284.
Malkova NV, Collin ZY, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16.
Witt RM, Galligan MM, Despinoy JR, Segal R. Olfactory behavioral testing in the adult mouse. JoVE. 2009;23:949.
Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28:6664–9.
Pereira AG, Moita MA. Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr Opin Neurobiol. 2016;41:179–87.
Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72.
Blomeley C, Garau C, Burdakov D. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat Neurosci. 2018;21:29.
Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28.
Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, et al. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell. 2015;163:1153–64.
Funding and disclosure
Research experiments were funded by NIH grants P60 AA011605 (TLK), R01 AA019454 (TLK), U01 AA020911 (TLK), T32 AA007573 (LSH), and F32 AA026485 (MMP). The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
About this article
Cite this article
Hwa, L.S., Neira, S., Pina, M.M. et al. RETRACTED ARTICLE: Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacol 44, 766–775 (2019). https://doi.org/10.1038/s41386-018-0279-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0279-2
This article is cited by
-
Circuit- and laminar-specific regulation of medial prefrontal neurons by chronic stress
Cell & Bioscience (2023)
-
Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: a systematic review
Environmental Evidence (2023)
-
Prefrontal engrams of long-term fear memory perpetuate pain perception
Nature Neuroscience (2023)
-
Insular cortex corticotropin-releasing factor integrates stress signaling with social affective behavior
Neuropsychopharmacology (2022)
-
Prelimbic cortex glucocorticoid receptors regulate the stress-mediated inhibition of pain contagion in male mice
Neuropsychopharmacology (2021)