Abstract
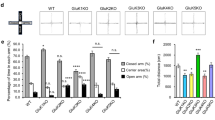
NETO1 and NETO2 are auxiliary subunits of kainate receptors (KARs). They interact with native KAR subunits to modulate multiple aspects of receptor function. Variation in KAR genes has been associated with psychiatric disorders in humans, and in mice, knockouts of the Grik1 gene have increased, while Grik2 and Grik4 knockouts have reduced anxiety-like behavior. To determine whether the NETO proteins regulate anxiety and fear through modulation of KARs, we undertook a comprehensive behavioral analysis of adult Neto1−/− and Neto2−/− mice. We observed no differences in anxiety-like behavior. However, in cued fear conditioning, Neto2−/−, but not Neto1−/− mice, showed higher fear expression and delayed extinction compared to wild type mice. We established, by in situ hybridization, that Neto2 was expressed in both excitatory and inhibitory neurons throughout the fear circuit including the medial prefrontal cortex, amygdala, and hippocampus. Finally, we demonstrated that the relative amount of synaptosomal KAR GLUK2/3 subunit was 20.8% lower in the ventral hippocampus and 36.5% lower in the medial prefrontal cortex in Neto2−/− compared to the Neto2+/+ mice. The GLUK5 subunit abundance was reduced 23.8% in the ventral hippocampus and 16.9% in the amygdala. We conclude that Neto2 regulates fear expression and extinction in mice, and that its absence increases conditionability, a phenotype related to post-traumatic stress disorder and propose that this phenotype is mediated by reduced KAR subunit abundance at synapses of fear-associated brain regions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41.
Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–96.
Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci. 2011;14:866–73.
Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, et al. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci. 2011;31:10009–18.
Tang M, Ivakine E, Mahadevan V, Salter MW, McInnes RR. Neto2 interacts with the scaffolding protein GRIP and regulates synaptic abundance of kainate receptors. PLoS ONE. 2012;7:e51433.
Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, McBain CJ. Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J Neurosci. 2014;34:622–8.
Ivakine EA, Acton BA, Mahadevan V, Ormond J, Tang M, Pressey JC, et al. Neto2 is a KCC2 interacting protein required for neuronal Cl- regulation in hippocampal neurons. Proc Natl Acad Sci USA. 2013;110:3561–6.
Wyeth MS, Pelkey KA, Yuan X, Vargish G, Johnston AD, Hunt S, et al. Neto auxiliary subunits regulate interneuron somatodendritic and presynaptic kainate receptors to control network inhibition. Cell Rep. 2017;20:2156–68.
Orav E, Atanasova T, Shintyapina A, Kesaf S, Kokko M, Partanen J, et al. NETO1 Guides Development of Glutamatergic Connectivity in the Hippocampus by Regulating Axonal Kainate Receptors. eNeuro. 2017;4:ENEURO.0048-17.2017.
Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–98.
Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci. 2000;20:196–205.
Watanabe-Iida I, Konno K, Akashi K, Abe M, Natsume R, Watanabe M, et al. Determination of kainate receptor subunit ratios in mouse brain using novel chimeric protein standards. J Neurochem. 2016;136:295–305.
Wondolowski J, Frerking M. Subunit-dependent postsynaptic expression of kainate receptors on hippocampal interneurons in area CA1. J Neurosci. 2009;29:563–74.
Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, et al. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709.
Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, et al. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2007;104:12181–6.
Lauri SE, Segerstrale M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, et al. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci. 2005;25:4473–84.
Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–82.
Kidd FL, Coumis U, Collingridge GL, Crabtree JW, Isaac JT. A presynaptic kainate receptor is involved in regulating the dynamic properties of thalamocortical synapses during development. Neuron. 2002;34:635–46.
Gratacos M, Costas J, de Cid R, Bayes M, Gonzalez JR, Baca-Garcia E, et al. Identification of new putative susceptibility genes for several psychiatric disorders by association analysis of regulatory and non-synonymous SNPs of 306 genes involved in neurotransmission and neurodevelopment. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:808–16.
Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2015;20:337–44.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–902.
Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS ONE. 2007;2:e167.
Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–72.
Catches JS, Xu J, Contractor A. Genetic ablation of the GluK4 kainate receptor subunit causes anxiolytic and antidepressant-like behavior in mice. Behav Brain Res. 2012;228:406–14.
Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31.
Laine MA, Trontti K, Misiewicz Z, Sokolowska E, Kulesskaya N, Heikkinen A, et al. Genetic Control of Myelin Plasticity after Chronic Psychosocial Stress. eNeuro. 2018;5:ENEURO.0166-18.2018.
Fitzgerald PJ, Pinard CR, Camp MC, Feyder M, Sah A, Bergstrom HC, et al. Durable fear memories require PSD-95. Mol Psychiatry. 2015;20:901–12.
Dominguez G, Dagnas M, Decorte L, Vandesquille M, Belzung C, Beracochea D, et al. Rescuing prefrontal cAMP-CREB pathway reverses working memory deficits during withdrawal from prolonged alcohol exposure. Brain Struct Funct. 2016;221:865–77.
Lahti L, Haugas M, Tikker L, Airavaara M, Voutilainen MH, Anttila J, et al. Differentiation and molecular heterogeneity of inhibitory and excitatory neurons associated with midbrain dopaminergic nuclei. Development. 2016;143:516–29.
Maccarrone G, Filiou MD. Protein profiling and phosphoprotein analysis by isoelectric focusing. Methods Mol Biol. 2015;1295:293–303.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:11.
Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31.
Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–32.
Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014;69:8 31 1–17.
Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Res Bull. 2002;58:517–27.
Kirkby RJ, Stein DG, Kimble RJ, Kimble DP. Effects of hippocampal lesions and duration of sensory input on spontaneous alternation. J Comp Physiol Psychol. 1967;64:342–5.
Divac I, Wikmark R, Gade A. Spontaneous alternation in rats with lesions in the frontal lobe: an extension of the frontal lobe syndrome. Physiol Psychol. 1975;3:7.
LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–29.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82.
Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–5.
Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–94.
Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–33.
Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–8.
Wegerer M, Blechert J, Kerschbaum H, Wilhelm FH. Relationship between fear conditionability and aversive memories: evidence from a novel conditioned-intrusion paradigm. PLoS ONE. 2013;8:e79025.
Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–54.
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19.
Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85.
Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–4.
Marek R, Xu L, Sullivan RKP, Sah P. Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nat Neurosci. 2018;21:654–8.
Jaskolski F, Coussen F, Mulle C. Subcellular localization and trafficking of kainate receptors. Trends Pharmacol Sci. 2005;26:20–6.
Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. Ed 3. New York, NY: Academic Press; 2008.
Acknowledgements
We thank Roderick R. McInnes for Neto1 and Neto2 knockout mice and antibody for NETO2, Saija-Anita Callan for help in behavioral testing, Marijiana Kanisek for performing of MWM test, and Sari Lauri, Ester Orav, Sebnem Kesaf, Anna Kirjavainen, Laura Tikker, and Hovatta lab members for helpful discussions. We thank the Mouse Behavioral Phenotype Facility (MBPF) supported by Biocenter Finland and Helsinki Institute of Life Science, and the Biocomplex Unit (Instruct Center for Virus and Macromolecular Complex Production, ICVIR 2009-2017) for the use of their facilities, and Sari Korhonen from ICVIR for technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mennesson, M., Rydgren, E., Lipina, T. et al. Kainate receptor auxiliary subunit NETO2 is required for normal fear expression and extinction. Neuropsychopharmacol. 44, 1855–1866 (2019). https://doi.org/10.1038/s41386-019-0344-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-019-0344-5
This article is cited by
-
α5-nAChR/NETO2 contributed to chronic stress-promoted lung adenocarcinoma progression
Cancer Cell International (2025)
-
Heightened fear in the absence of the kainate receptor auxiliary subunit NETO2: implications for PTSD
Neuropsychopharmacology (2019)