Abstract
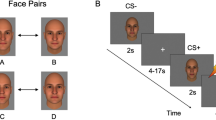
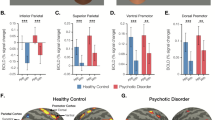
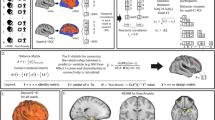
Social impairment occurs across the psychosis spectrum, but its pathophysiology remains poorly understood. Here we tested the hypothesis that reduced differential responses (aversive vs. neutral) in neural circuitry underpinning aversive conditioning of social stimuli characterizes the psychosis spectrum. Participants age 10–30 included a healthy control group (HC, analyzed n = 36) and a psychosis spectrum group (PSY, n = 71), including 49 at clinical risk for psychosis and 22 with a frank psychotic disorder. 3T fMRI utilized a passive aversive conditioning paradigm, with neutral faces as conditioned stimuli (CS) and a scream as the unconditioned stimulus. fMRI conditioning was indexed as the activation difference between aversive and neutral trials. Analysis focused on amygdala, ventromedial prefrontal cortex, and anterior insula, regions previously implicated in aversive and social-emotional processing. Ventromedial prefrontal cortex activated more to neutral than aversive CS; this “safety effect” was driven by HC and reduced in PSY, and correlated with subjective emotional ratings following conditioning. Insula showed the expected aversive conditioning effect, and although no group differences were found, its activation in PSY correlated with anxiety severity. Unexpectedly, amygdala did not show aversive conditioning; its activation trended greater for neutral than aversive CS, and did not differ significantly based on group or symptom severity. We conclude that abnormalities in social aversive conditioning are present across the psychosis spectrum including clinical risk, linked to a failure of safety processing. Aversive and safety learning provide translational paradigms yielding insight into pathophysiology of psychosis risk, and providing potential targets for therapeutic and preventative interventions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bottlender R, Strauss A, Moller HJ. Social disability in schizophrenic, schizoaffective and affective disorders 15 years after first admission. Schizophr Res. 2010;116:9–15.
Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14–24.
Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9.
Pinkham AE, Harvey PD, Penn DL. Paranoid individuals with schizophrenia show greater social cognitive bias and worse social functioning than non-paranoid individuals with schizophrenia. Schizophr Res Cogn. 2016;3:33–38.
Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–31.
Ballon JS, Kaur T, Marks II, Cadenhead KS. Social functioning in young people at risk for schizophrenia. Psychiatry Res. 2007;151:29–35.
Lyne J, O’Donoghue B, Owens E, Renwick L, Madigan K, Kinsella A, et al. Prevalence of item level negative symptoms in first episode psychosis diagnoses. Schizophr Res. 2012;135:128–33.
Yung AR. Toward improved risk prediction in individuals at high risk of psychotic disorders. Am J Psychiatry. 2015;172:932–3.
Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. September 2018. https://doi.org/10.1016/j.neubiorev.2018.09.012.
Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38:608–21.
Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–66.
Pinkham AE, Gur RE, Gur RC. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Rev Neurother. 2007;7:807–16.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Finlayson K, Lampe JF, Hintze S, Wurbel H, Melotti L. Facial indicators of positive emotions in rats. PLoS ONE. 2016;11:e0166446.
Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94.
Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–33.
Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–8.
Harrison BJ, Fullana MA, Via E, Soriano-Mas C, Vervliet B, Martinez-Zalacain I, et al. Human ventromedial prefrontal cortex and the positive affective processing of safety signals. Neuroimage. 2017;152:12–18.
Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–9.
Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903.
Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–36.
Romaniuk L, Honey GD, King JR, Whalley HC, McIntosh AM, Levita L, et al. Midbrain activation during Pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–54.
Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–63.
Linnman C, Coombs G III, Goff DC, Holt DJ. Lack of insula reactivity to aversive stimuli in schizophrenia. Schizophr Res. 2013;143:150–7.
Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, et al. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2015;72:456–65.
Bourque J, Spechler PA, Potvin S, Whelan R, Banaschewski T, Bokde ALW, et al. Functional neuroimaging predictors of self-reported psychotic symptoms in adolescents. Am J Psychiatry. 2017;174:566–75.
van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95.
Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305.
Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56:1356–69.
Watters AJ, Rupert PE, Wolf DH, Calkins ME, Gur RC, Gur RE, et al. Social aversive conditioning in youth at clinical high risk for psychosis and with psychosis: an ERP study. Schizophr Res. 2018;202:291–6.
Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–57.
Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–25.
Greco JA, Liberzon I. Neuroimaging of fear-associated learning. Neuropsychopharmacology. 2016;41:320–34.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98.
Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113:7900–5.
Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J Neurosci. 2016;36:5047–54.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–84.
Park IH, Park HJ, Chun JW, Kim EY, Kim JJ. Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neurosci Lett. 2008;440:119–24.
Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child Adolesc Psychiatr Clin N Am. 2013;22:689–714.
Koutsouleris N, Patschurek-Kliche K, Scheuerecker J, Decker P, Bottlender R, Schmitt G, et al. Neuroanatomical correlates of executive dysfunction in the at-risk mental state for psychosis. Schizophr Res. 2010;123:160–74.
Lataster J, Collip D, Ceccarini J, Hernaus D, Haas D, Booij L, et al. Familial liability to psychosis is associated with attenuated dopamine stress signaling in ventromedial prefrontal cortex. Schizophr Bull. 2014;40:66–77.
LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38.
Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865.
Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123.
Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, Shafer AT, Baron CA, Seres P, et al. Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 2016;133:98–110.
Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19:1636–46.
Boll S, Gamer M, Gluth S, Finsterbusch J, Buchel C. Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur J Neurosci. 2013;37:758–67.
Wolf DH, Satterthwaite TD, Loughead J, Pinkham A, Overton E, Elliott MA, et al. Amygdala abnormalities in first-degree relatives of individuals with schizophrenia unmasked by benzodiazepine challenge. Psychopharmacology (Berl). 2011;218:503–12.
Roalf DR, Nanga RP, Rupert PE, Hariharan H, Quarmley M, Calkins ME, et al. Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum. Mol Psychiatry. 2017;22:1298–305.
Acknowledgements
The authors thank Karthik Prabhakaran for technical assistance, and Conte Summer Undergraduate Program (C-SURE) students Joanna Kass, Lauren Carpenter, and Phillip Dmitriev for assistance with data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Quarmley, M., Gur, R.C., Turetsky, B.I. et al. Reduced safety processing during aversive social conditioning in psychosis and clinical risk. Neuropsychopharmacol. 44, 2247–2253 (2019). https://doi.org/10.1038/s41386-019-0421-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-019-0421-9
This article is cited by
-
Direct serotonin release in humans shapes aversive learning and inhibition
Nature Communications (2024)
-
Changes in responses of the amygdala and hippocampus during fear conditioning are associated with persecutory beliefs
Scientific Reports (2024)
-
Impairment in acquisition of conditioned fear in schizophrenia
Neuropsychopharmacology (2022)
-
BDNF haploinsufficiency induces behavioral endophenotypes of schizophrenia in male mice that are rescued by enriched environment
Translational Psychiatry (2021)