Abstract
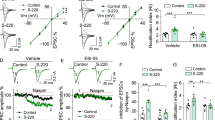
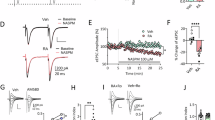
Cue-induced drug craving progressively intensifies after withdrawal from self-administration of cocaine, methamphetamine, and other drugs of abuse, a phenomenon termed incubation of craving. For cocaine and methamphetamine, expression of incubated craving ultimately depends on strengthening of nucleus accumbens (NAc) synapses through an accumulation of high conductance Ca2+-permeable AMPA receptors (CP-AMPARs) that is detectable with electrophysiological approaches. This study sought to further characterize glutamate receptor adaptations in NAc core during methamphetamine incubation. Previous biochemical studies revealed that the CP-AMPARs accumulating after cocaine incubation are mainly homomeric GluA1 receptors and that their accumulation is reflected by increased cell surface GluA1. Here, for methamphetamine, we observed no significant change in surface or total GluA1 (GluA2 and GluA3 were also unchanged). Nonetheless, GluA1 translation was elevated after incubation of methamphetamine craving, as recently found for cocaine. Additionally, for cocaine, we previously observed a withdrawal-dependent decrease in mGlu1 surface expression that precedes and enables CP-AMPAR accumulation and incubation of craving, reflecting weakening of mGlu1-dependent mechanisms that normally limit synaptic CP-AMPAR levels in the NAc core. Here, we observed no change in surface or total mGlu1 protein or its coupling to Homer scaffolding proteins after methamphetamine withdrawal, nor did elevation of mGlu1 tone through repeated injections of an mGlu1-positive allosteric modulator delay incubation of craving. These findings suggest a common role for increased GluA1 translation, but not decreased mGlu1 function, in the incubation of methamphetamine and cocaine craving. We speculate that increased GluA1 translation near synapses may drive formation and synaptic insertion of homomeric GluA1 receptors in the absence of detectable changes in GluA1 protein levels.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brecht M-L, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20.
Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–26.
Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–9.
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol Psychiatry. 2016;0:5–15.
Adhikary S, Caprioli D, Venniro M, Kallenberger P, Shaham Y, Bossert JM. Incubation of extinction responding and cue-induced reinstatement, but not context- or drug priming-induced reinstatement, after withdrawal from methamphetamine. Addict Biol. 2017;22:977–90.
Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25-52. https://doi.org/10.1016/bs.pbr.2015.08.004.
Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS ONE. 2013;8:e68791.
Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine Experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21.
Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–65.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+- permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–51.
Stefanik MT, Milovanovic M, Werner CT, Spainhour JCG, Wolf ME. Withdrawal from cocaine self-administration alters the regulation of protein translation in the nucleus accumbens. Biol Psychiatry. 2018;84:223–32.
McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–41.
Scheyer AF, Christian DT, Wolf M, Tseng KY. Emergence of endocytosis-dependent mGlu1 LTD at nucleus accumbens synapses after withdrawal from cocaine self-administration. Front Synaptic Neurosci. 2018;10:36.
Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67.
Halbout B, Bernardi RE, Hansson AC, Spanagel R. Incubation of cocaine seeking following brief cocaine experience in mice is enhanced by mGluR1 blockade. J Neurosci. 2014;34:1781–90.
Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic glutamate receptor. Curr Opin Neurobiol. 2003;13:271–8.
McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–43.
Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72.
Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–7.
Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev. 2013;41:107–15.
Starck SR, Green HM, Alberola-Ila J, Roberts RW. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem Biol. 2004;11:999–1008.
Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609.
Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharm. 2008;75:112–33.
Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49.
Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014;97:37–44.
Rivière GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharm Exp Ther. 2000;292:1042–7.
Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93.
Taheri S, Xun Z, See RE, Joseph JE, Reichel CM. Cocaine and methamphetamine induce opposing changes in BOLD signal response in rats. Brain Res. 2016;1642:497–504.
Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39.
Mishra D, Pena-Bravo JI, Leong K-C, Lavin A, Reichel CM. Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct Funct. 2017;222:2031–9.
Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–22.
Schwendt M, Reichel CM, See RE, Hashimoto K. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. 2012. https://doi.org/10.1371/journal.pone.0034299.
Graves SM, Clark MJ, Traynor JR, Hu X-T, Napier TC. Nucleus accumbens shell excitability is decreased by methamphetamine self-administration and increased by 5-HT2C receptor inverse agonism and agonism. Neuropharmacology. 2015;89:113–21.
Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–6.
Loweth JA, Reimers JM, Caccamise A, Stefanik MT, Woo K-Y, Chauhan NM, et al. mGlu1 tonically regulates levels of calcium-permeable AMPA receptors in cultured nucleus accumbens neurons through retinoic acid signaling and protein translation. Eur J Neurosci. 2018. https://doi.org/10.1111/ejn.14151.
Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–16.
Scheyer AF, Wolf ME, Tseng KY. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J Neurosci. 2014;34:3095–3100.
Werner CT, Stefanik MT, Milovanovic M, Caccamise A, Wolf ME. Protein translation in the nucleus accumbens is dysregulated during cocaine withdrawal and required for expression of incubation of cocaine craving. J Neurosci. 2018;38:2683–97.
Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015;40:1297–306.
Shin CB, Templeton TJ, Chiu AS, Kim J, Gable ES, Vieira PA, et al. Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology. 2018;128:293–300.
Cates HM, Li X, Purushothaman I, Kennedy PJ, Shen L, Shaham Y, et al. Genome-wide transcriptional profiling of central amygdala and orbitofrontal cortex during incubation of methamphetamine craving. Neuropsychopharmacology. 2018. https://doi.org/10.1038/s41386-018-0158-x.
Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015;35:8232–44.
Li X, Carreria MB, Witonsky KR, Zeric T, Lofaro OM, Bossert JM, et al. Role of dorsal striatum histone deacetylase 5 in incubation of methamphetamine craving. Biol Psychiatry. 2018;84:213–22.
Li X, Witonsky KR, Lofaro OM, Surjono F, Zhang J, Bossert JM, et al. Role of anterior intralaminar nuclei of thalamus projections to dorsomedial striatum in incubation of methamphetamine craving. J Neurosci. 2018;38:2270–82.
Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41.
Acknowledgements
We thank Dr. Katherine Roche for generously supplying the GluA2/3 antibody and Dr. Pamela Metten for advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Murray, C.H., Loweth, J.A., Milovanovic, M. et al. AMPA receptor and metabotropic glutamate receptor 1 adaptations in the nucleus accumbens core during incubation of methamphetamine craving. Neuropsychopharmacol. 44, 1534–1541 (2019). https://doi.org/10.1038/s41386-019-0425-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-019-0425-5
This article is cited by
-
Dopamine dysfunction in stimulant use disorders: mechanistic comparisons and implications for treatment
Molecular Psychiatry (2022)
-
Footshock-Induced Abstinence from Compulsive Methamphetamine Self-administration in Rat Model Is Accompanied by Increased Hippocampal Expression of Cannabinoid Receptors (CB1 and CB2)
Molecular Neurobiology (2022)
-
Getting to the core of relapse: The role of the nucleus accumbens core in the incubation of methamphetamine seeking after choice-based abstinence
Neuropsychopharmacology (2020)