Abstract
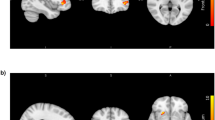
Second-generation antipsychotic drugs (SGAs) are essential in the treatment of psychotic disorders, but are well-known for inducing substantial weight gain and obesity. Critically, weight gain may reduce life expectancy for up to 20–30 years in patients with psychotic disorders, and prognostic biomarkers are generally lacking. Even though other receptors are also implicated, the dorsal striatum, rich in dopamine D2 receptors, which are antagonized by antipsychotic medications, plays a key role in the human reward system and in appetite regulation, suggesting that altered dopamine activity in the striatal reward circuitry may be responsible for increased food craving and weight gain. Here, we measured striatal volume and striatal resting-state functional connectivity at baseline, and weight gain over the course of 12 weeks of antipsychotic treatment in 81 patients with early-phase psychosis. We also included a sample of 58 healthy controls. Weight measurements were completed at baseline, and then weekly for 4 weeks, and every 2 weeks until week 12. We used linear mixed models to compute individual weight gain trajectories. Striatal volume and whole-brain striatal connectivity were then calculated for each subject, and used to assess the relationship between striatal structure and function and individual weight gain in multiple regression models. Patients had similar baseline weights and body mass indices (BMI) compared with healthy controls. There was no evidence that prior drug exposure or duration of untreated psychosis correlated with baseline BMI. Higher left putamen volume and lower sensory motor connectivity correlated with the magnitude of weight gain in patients, and these effects multiplied when the structure–function interaction was considered in an additional exploratory analysis. In conclusion, these results provide evidence for a correlation of striatal structure and function with antipsychotic-induced weight gain. Lower striatal connectivity was associated with more weight gain, and this relationship was stronger for higher compared with lower left putamen volumes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orrey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62.
Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Exp Opin Drug Saf. 2015;14:73–96.
Kapur S, Marques T. Dopamine, striatum, antipsychotics, and questions about weight gain. JAMA Psychiatry. 2016;73:107–8.
Winkelbeiner S, Leucht S, Kane JM, Homan P. Evaluation of differences in individual treatment response in schizophrenia spectrum disorders: a meta-analysis. JAMA Psychiatry. 2019; Jun 3. doi: 10.1001/jamapsychiatry.2019.1530.
Malhotra AK, Correll CU, Chowdhury NI, Muller DJ, Gregersen PK, Lee AT, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch Gen psychiatry. 2012;69:904–12.
Brandl EJ, Tiwari AK, Zai CC, Nurmi EL, Chowdhury NI, Arenovich T, et al. Genome-wide association study on antipsychotic-induced weight gain in the catie sample. Pharm J. 2015;16:352–6.
Zhang J-P, Lencz T, Zhang RX, Nitta M, Maayan L, John M, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42:1418–37.
Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46.
Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain. CNS Drugs. 2011;25:1035–59.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4.
Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–15.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21.
Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS ONE. 2012;7:e31089.
Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–9.
Nielsen M, Rostrup E, Wulff S, Glenthoj B, Ebdrup BH. Striatal reward activity and antipsychotic-associated weight change in patients with schizophrenia undergoing initial treatment. JAMA Psychiatry. 2016;73:121–8.
Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, et al. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69:1226.
Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang J-P, et al. A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015;41:1227–36.
Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–9.
Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–24.
Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–61.
Mole TB, Mak E, Chien Y, Voon V. Dissociated accumbens and hippocampal structural abnormalities across obesity and alcohol dependence. Int J Neuropsychopharmacol. 2016;19:pyw039.
Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, et al. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci Biobehav Rev. 2018;94:271–85.
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23.
Homan P, Argyelan M, DeRosse P, Szeszko PR, Gallego JA, Hanna L, et al. Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology. 2019;44:915–22.
Robinson, DG, Gallego, JA, John, M, Hanna, LA, Zhang, J-P, Birnbaum, ML et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: results from a 16-week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. 2018;204:295–303.
Sarpal DK, Robinson DG, Fales C, Lencz T, Argyelan M, Karlsgodt KH, et al. Relationship between duration of untreated psychosis and intrinsic corticostriatal connectivity in patients with early phase schizophrenia. Neuropsychopharmacology. 2017;42:2214–21.
Hedeker D, Gibbons RD. Longitudinal data analysis. Volume 451. Hoboken, NJ: John Wiley & Sons; 2006.
Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage. 2017;154:174–87.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (compcor) for bold and perfusion based fMRI. NeuroImage. 2007;37:90–101.
Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using acompcor. NeuroImage. 2014;96:22–35.
Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–47.
Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201.
Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fmri. NeuroImage. 2015;105:536–51.
Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5.
Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia. Arch Gen Psychiatry. 1999;56:133.
Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004;21:1563–75.
Steen RG, Mull C, Mcclure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia. Br J Psychiatry. 2006;188:510–8.
Nielsen MO, Rostrup E, Wulff S, Bak N, Broberg BV, Lublin H, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69:1195.
Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, et al. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol Psychiatry. 2012;71:898–905.
Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res. 2002;53:925–33.
Thakore JH, Mann JN, Vlahos I, Martin A, Reznek R. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes. 2002;26:137–41.
Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry. 2004;184(S47):s64–66.
Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–120.
Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–9.
Vikdahl M, Carlsson M, Linder J, Forsgren L, Haglin L. Weight gain and increased central obesity in the early phase of parkinson’s disease. Clin Nutr. 2014;33:1132–9.
Cota D, Barrera JG, Seeley RJ. Leptin in energy balance and reward: two faces of the same coin? Neuron. 2006;51:678–80.
Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc B: Biol Sci. 2006;361:1149–58.
Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D, et al. Nonhedonic food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–80.
Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6.
Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–43.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60.
Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by taqia a1 allele. Science. 2008;322:449–52.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–47.
Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci USA. 2016;114:160–65.
Contreras-Rodríguez O, Martín-Pérez C, Vilar-López R, Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biol Psychiatry. 2017;81:789–96.
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40.
Meltzer HY. New trends in the treatment of schizophrenia. CNS Neurol Disord—Drug Targets. 2017;16:900–6.
Rasmussen H, Ebdrup BH, Oranje B, Pinborg LH, Knudsen GM, Glenthoj B. Neocortical serotonin2a receptor binding predicts quetiapine associated weight gain in antipsychotic-naive first-episode schizophrenia patients. Int J Neuropsychopharmacol. 2014;17:1729–36.
Trampush JW, Lencz T, DeRosse P, John M, Gallego JA, Petrides G, et al. Relationship of cognition to clinical response in first-episode schizophrenia spectrum disorders. Schizophr Bull. 2015;41:1237–47.
Acknowledgements
The authors thank Dr Lauren Hanna and Dr Juan Gallego for their careful clinical oversight of the study. They acknowledge their patients, their patients’ families, and their psychiatry research support staff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Homan, P., Argyelan, M., Fales, C.L. et al. Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacol. 44, 1948–1954 (2019). https://doi.org/10.1038/s41386-019-0464-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-019-0464-y
This article is cited by
-
Exploratory analysis of the relationship between striatal connectivity and apathy during phosphodiesterase 10 inhibition in schizophrenia: findings from a randomized crossover trial
BMC Medicine (2025)
-
Associations between antipsychotics-induced weight gain and brain networks of impulsivity
Translational Psychiatry (2024)
-
Unveiling Promising Neuroimaging Biomarkers for Schizophrenia Through Clinical and Genetic Perspectives
Neuroscience Bulletin (2024)
-
Interaction between baseline BMI and baseline disease severity predicts greater improvement in negative symptoms in first-episode schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Mechanism and treatments of antipsychotic-induced weight gain
International Journal of Obesity (2023)