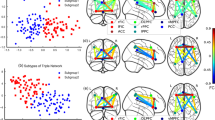
Abstract
Patients with schizophrenia (SCZ), as well as their unaffected siblings (SIB), show functional connectivity (FC) alterations during performance of tasks involving attention. As compared with SCZ, these alterations are present in SIB to a lesser extent and are more pronounced during high cognitive demand, thus possibly representing one of the pathways in which familial risk is translated into the SCZ phenotype. Our aim is to measure the separability of SCZ and SIB from healthy controls (HC) using attentional control-dependent FC patterns, and to test to which extent these patterns span a continuum of neurofunctional alterations between HC and SCZ. 65 SCZ with 65 age and gender-matched HC and 39 SIB with 39 matched HC underwent the Variable Attentional Control (VAC) task. Load-dependent connectivity matrices were generated according to correct responses in each VAC load. Classification performances of high, intermediate and low VAC load FC on HC-SCZ and HC-SIB cohorts were tested through machine learning techniques within a repeated nested cross-validation framework. HC-SCZ classification models were applied to the HC-SIB cohort, and vice-versa. A high load-related decreased FC pattern discriminated between HC and SCZ with 66.9% accuracy and with 57.7% accuracy between HC and SIB. A high load-related increased FC network separated SIB from HC (69.6% accuracy), but not SCZ from HC (48.5% accuracy). Our findings revealed signatures of attentional FC abnormalities shared by SCZ and SIB individuals. We also found evidence for potential, SIB-specific FC signature, which may point to compensatory neurofunctional mechanisms in persons at familial risk for schizophrenia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bertolino A, Blasi G. The genetics of schizophrenia. Neuroscience. 2009;164:288–99.
Murray RM, Bhavsar V, Tripoli G, Howes O. 30 years on: how the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophrenia Bull. 2017;43:1190–6.
Antonucci LA, Di Carlo P, Passiatore R, Papalino M, Monda A, Amoroso N, et al. Thalamic connectivity measured with fMRI is associated with a polygenic index predicting thalamo-prefrontal gene co-expression. Brain Struct Funct. 2019 Apr;224:1331–44.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Antonucci LA, Taurisano P, Fazio L, Gelao B, Romano R, Quarto T, et al. Association of familial risk for schizophrenia with thalamic and medial prefrontal functional connectivity during attentional control. Schizophrenia Res. 2016;173:23–9.
Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, et al. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20:837–45.
MacDonald AW 3rd, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients' nonpsychotic relatives. Schizophrenia Bull. 2009;35:1142–62.
Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75.
Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci USA. 1967;58:199–205.
Smieskova R, Marmy J, Schmidt A, Bendfeldt K, Riecher-Rssler A, Walter M, et al. Do subjects at clinical high risk for psychosis differ from those with a genetic high risk?-A systematic review of structural and functional brain abnormalities. Curr Med Chem. 2013;20:467–81.
Jiang T, Zhou Y, Liu B, Liu Y, Song M. Brainnetome-wide association studies in schizophrenia: the advances and future. Neurosci Biobehav Rev. 2013;37:2818–35.
Orban P, Desseilles M, Mendrek A, Bourque J, Bellec P, Stip E. Altered brain connectivity in patients with schizophrenia is consistent across cognitive contexts. J Psychiatry Neurosci. 2017;42:17–26.
Wei Y, Chang M, Womer FY, Zhou Q, Yin Z, Wei S, et al. Local functional connectivity alterations in schizophrenia, bipolar disorder, and major depressive disorder. Journal of affective disorders. 2018;236:266–73.
Wu XJ, Zeng LL, Shen H, Yuan L, Qin J, Zhang P, et al. Functional network connectivity alterations in schizophrenia and depression. Psychiatry Res Neuroimaging. 2017;263:113–20.
Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, et al. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014;1562:87–99.
Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–66.
Peeters SC, van de Ven V, Gronenschild EH, Patel AX, Habets P, Goebel R, et al. Default mode network connectivity as a function of familial and environmental risk for psychotic disorder. PLoS One. 2015;10:e0120030.
Poppe AB, Carter CS, Minzenberg MJ, MacDonald AW 3rd. Task-based functional connectivity as an indicator of genetic liability to schizophrenia. Schizophrenia Res. 2015;162:118–23.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1279–84.
Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–9.
Dwyer DB, Falkai P, Koutsouleris N. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 2018;14:91–118.
Lessov-Schlaggar CN, Rubin JB, Schlaggar BL. The fallacy of univariate solutions to complex systems problems. Front Neurosci. 2016;10:267.
Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75:746–8.
Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Therapeutics. 2001;69:89–95.
Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. NeuroImage. 2004;23:21–24.
Koutsouleris N, Riecher-Rossler A, Meisenzahl EM, Smieskova R, Studerus E, Kambeitz-Ilankovic L, et al. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophrenia Bull. 2015;41:471–82.
Lao Z, Shen D, Xue Z, Karacali B, Resnick SM, Davatzikos C. Morphological classification of brains via high-dimensional shape transformations and machine learning methods. NeuroImage. 2004;21:46–57.
Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–52.
Iniesta R, Stahl D, McGuffin P. Machine learning, statistical learning and the future of biological research in psychiatry. Psychological Med. 2016;46:2455–65.
Cabral C, Kambeitz-Ilankovic L, Kambeitz J, Calhoun VD, Dwyer DB, von Saldern S, et al. Classifying schizophrenia using multimodal multivariate pattern recognition analysis: evaluating the impact of individual clinical profiles on the neurodiagnostic performance. Schizophrenia Bull. 2016;42(Suppl 1):S110–7.
Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology 2015;40:1742–51.
Bendfeldt K, Smieskova R, Koutsouleris N, Kloppel S, Schmidt A, Walter A, et al. Classifying individuals at high-risk for psychosis based on functional brain activity during working memory processing. NeuroImage Clin. 2015;9:555–63.
Liu M, Zeng LL, Shen H, Liu Z, Hu D. Potential risk for healthy siblings to develop schizophrenia: evidence from pattern classification with whole-brain connectivity. Neuroreport. 2012;23:265–9.
Yu Y, Shen H, Zhang H, Zeng LL, Xue Z, Hu D. Functional connectivity-based signatures of schizophrenia revealed by multiclass pattern analysis of resting-state fMRI from schizophrenic patients and their healthy siblings. Biomed Eng. 2013;12:10.
Wang J, Cao H, Liao Y, Liu W, Tan L, Tang Y, et al. Three dysconnectivity patterns in treatment-resistant schizophrenia patients and their unaffected siblings. NeuroImage Clinical. 2015;8:95–103.
Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–71.
Zhu DC, Tarumi T, Khan MA, Zhang R. Vascular coupling in resting-state fMRI: evidence from multiple modalities. J Cereb Blood Flow Metab. 2015;35:1910–20.
van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, Hofman D, et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2015;126:1468–81.
Pearlson GD, Calhoun VD. Convergent approaches for defining functional imaging endophenotypes in schizophrenia. Front Hum Neurosci. 2009;3:37.
Linscott RJ, Morton SE, Investigators G. The latent taxonicity of schizotypy in biological siblings of probands with schizophrenia. Schizophrenia Bull. 2018;44:922–32.
Pearl J. The foundations of causal inference. Soc Metodol. 2010;40:75–149.
Rosenbaum PR, Rubin, DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
First MB, GM, Spitzer RL, Williams JBW. Guide for the structured clinical interview for DSM-IV axis I disorders-research version. New York: Biometrics Research; 1996.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–76.
Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia Bull. 1991;17:555–64.
Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, et al. Preferential amygdala reactivity to the negative assessment of neutral faces. Biol Psychiatry. 2009;66:847–53.
Blasi G, Napolitano F, Ursini G, Di Giorgio A, Caforio G, Taurisano P, et al. Association of GSK-3beta genetic variation with GSK-3beta expression, prefrontal cortical thickness, prefrontal physiology, and schizophrenia. The American journal of psychiatry. 2013;170:868–76.
Gottlich M, Beyer F, Kramer UM. BASCO: a toolbox for task-related functional connectivity. Front Syst Neurosci. 2015;9:126.
Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105.
Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, Rosen M, Ruef A, Dwyer DB, et al. Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning analysis. JAMA Psychiatry. 2018;75:1156–72.
Wolpert DH. Stacked generalization. Neural Netw. 1992;5:241–59.
Koutsouleris N, Meisenzahl EM, Davatzikos C, Bottlender R, Frodl T, Scheuerecker J, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–12.
Hansen LK, Larsen J, Nielsen FA, Strother SC, Rostrup E, Savoy R, et al. Generalizable patterns in neuroimaging: how many principal components? NeuroImage. 1999;9:534–44.
Saeys Y, Inza I, Larranaga P. A review of feature selection techniques in bioinformatics. Bioinformatics. 2007;23:2507–17.
Vapnik VN. An overview of statistical learning theory. IEEE Trans Neural Netw. 1999;10:988–99.
Polikar R, Topalis A, Green D, Kounios J, Clark CM. Comparative multiresolution wavelet analysis of ERP spectral bands using an ensemble of classifiers approach for early diagnosis of Alzheimer's disease. Computers Biol Med. 2007;37:542–58.
Golland P, Fischl B. Permutation tests for classification: towards statistical significance in image-based studies. Inf Process Med imaging. 2003;18:330–41.
Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T, et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry. 2016;3:935–46.
Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
Modinos G, Pettersson-Yeo W, Allen P, McGuire PK, Aleman A, Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. NeuroImage. 2012;59:3033–41.
Shen H, Wang L, Liu Y, Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. NeuroImage. 2010;49:3110–21.
Blasi G, Goldberg TE, Elvevag B, Rasetti R, Bertolino A, Cohen J, et al. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25:594–602.
Kellermann T, Regenbogen C, De Vos M, Mossnang C, Finkelmeyer A, Habel U. Effective connectivity of the human cerebellum during visual attention. J Neurosc. 2012;32:11453–60.
Kizilirmak JM, Rosler F, Bien S, Khader PH. Inferior parietal and right frontal contributions to trial-by-trial adaptations of attention to memory. Brain Res. 2015;1614:14–27.
Brady RO, Jr, Gonsalvez I, Lee I, Ongur D, Seidman LJ, Schmahmann JD, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019:appiajp201818040429.
Nielsen JD, Madsen KH, Wang Z, Liu Z, Friston KJ, Zhou Y. Working memory modulation of frontoparietal network connectivity in first-episode schizophrenia. Cereb Cortex. 2017;27:3832–41.
Wang S, Zhang Y, Lv L, Wu R, Fan X, Zhao J, et al. Abnormal regional homogeneity as a potential imaging biomarker for adolescent-onset schizophrenia: a resting-state fMRI study and support vector machine analysis. Schizophrenia Res. 2018;192:179–84.
Fan Y, Gur RE, Gur RC, Wu X, Shen D, Calkins ME, et al. Unaffected family members and schizophrenia patients share brain structure patterns: a high-dimensional pattern classification study. Biol Psychiatry. 2008;63:118–24.
Moran ME, Hulshoff Pol H, Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain. 2013;136:3215–26.
Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. 2015;5:17275.
Guo W, Liu F, Xiao C, Yu M, Zhang Z, Liu J, et al. Increased causal connectivity related to anatomical alterations as potential endophenotypes for schizophrenia. Medicine. 2015;94:e1493.
Guo W, Liu F, Xiao C, Liu J, Yu M, Zhang Z, et al. Increased short-range and long-range functional connectivity in first-episode, medication-naive schizophrenia at rest. Schizophrenia Res. 2015;166:144–50.
Guo W, Liu F, Zhang Z, Liu G, Liu J, Yu L, et al. Increased cerebellar functional connectivity with the default-mode network in unaffected siblings of schizophrenia patients at rest. Schizophrenia Bull. 2015;41:1317–25.
Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biol Psychiatry. 2008;63:490–7.
Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–19.
Ventura J, Subotnik KL, Guzik LH, Hellemann GS, Gitlin MJ, Wood RC, et al. Remission and recovery during the first outpatient year of the early course of schizophrenia. Schizophrenia Res. 2011;132:18–23.
Schnack HG, Kahn RS. Detecting neuroimaging biomarkers for psychiatric disorders: sample size matters. Front Psychiatry. 2016;7:50.
Acknowledgements
We are grateful to Dr. Paolo Taurisano, Dr. Tiziana Quarto, Dr. Barbara Gelao, Dr. Raffaella Romano, for making data acquisition possible, and to Johanna Weiske for the methodological help.
Funding and Disclosure
This work was supported by the EU-FP7-HEALTH grant for the project “PRONIA” (Personalized Prognostic Tools for Early Psychosis Management-agreement number: 602152) and from the Structural European Funding of the Italian Minister of Education (Attraction and International Mobility–AIM-action, grant agreement No 1859959). The AIM action also funds LAA's salary. NK has received honoraria for two lectures from Otsuka. AB is a stockholder of Hoffmann-La Roche Ltd. He has also received lecture fees from Otsuka, Jannssen, Lundbeck, and consultant fees from Biogen. GB has received lecture fees by Janssen and Lundbeck. GP’s position is funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 798181. The funding bodies had no role in study design, data collection, and analysis, decision to publish, or preparation of the paper. This paper reflects only the author's views and the European Union is not liable for any use that may be made of the information contained therein. The remaning authors declare no biomedical financial interests and no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Antonucci, L.A., Penzel, N., Pergola, G. et al. Multivariate classification of schizophrenia and its familial risk based on load-dependent attentional control brain functional connectivity. Neuropsychopharmacol. 45, 613–621 (2020). https://doi.org/10.1038/s41386-019-0532-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-019-0532-3
This article is cited by
-
Classification of schizophrenia spectrum disorder using machine learning and functional connectivity: reconsidering the clinical application
BMC Psychiatry (2025)
-
Neuroplasticity of Speech-in-Noise Processing in Older Adults Assessed by Functional Near-Infrared Spectroscopy (fNIRS)
Brain Topography (2024)
-
Sampling inequalities affect generalization of neuroimaging-based diagnostic classifiers in psychiatry
BMC Medicine (2023)
-
Similarities and differences between multivariate patterns of cognitive and socio-cognitive deficits in schizophrenia, bipolar disorder and related risk
Schizophrenia (2023)
-
Hybrid brain model accurately predict human procrastination behavior
Cognitive Neurodynamics (2022)