Abstract
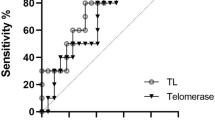
Individuals with severe psychiatric disorders have a reduced life expectancy compared to the general population. At the biological level, patients with these disorders present features that suggest the involvement of accelerated aging, such as increased circulating inflammatory markers and shorter telomere length (TL). To date, the role of the interplay between inflammation and telomere dynamics in the pathophysiology of severe psychiatric disorders has been scarcely investigated. In this study we measured T-lymphocytes TL with quantitative fluorescent in situ hybridization (Q-FISH) and plasma levels of inflammatory markers in a cohort comprised of 40 patients with bipolar disorder (BD), 41 with schizophrenia (SZ), 37 with major depressive disorder (MDD), and 36 non-psychiatric controls (NPC). TL was shorter in SZ and in MDD compared to NPC, while it was longer in BD (model F6, 137 = 20.128, p = 8.73 × 10−17, effect of diagnosis, F3 = 31.870; p = 1.08 × 10−15). There was no effect of the different classes of psychotropic medications, while duration of treatment with mood stabilizers was associated with longer TL (Partial correlation controlled for age and BMI: correlation coefficient = 0.451; p = 0.001). Levels of high-sensitivity C-Reactive Protein (hsCRP) were higher in SZ compared to NPC (adjusted p = 0.027), and inversely correlated with TL in the whole sample (r = −0.180; p = 0.042). Compared to NPC, patients with treatment resistant (TR) SZ had shorter TL (p = 0.001), while patients with TR MDD had higher levels of tumor necrosis factor-α (TNFα) compared to NPC (p = 0.028) and to non-TR (p = 0.039). Comorbidity with cardio-metabolic disorders did not influence the observed differences in TL, hsCRP, and TNFα among the diagnostic groups. Our study suggests that patients with severe psychiatric disorders present reduced TL and increased inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Who Mental Health. https://www.who.int/mental_health/management/en. Accessed 26 April 2020.
Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–8.
WHO mental disorders. https://www.who.int/news-room/fact-sheets/detail/mental-disorders. Accessed 25 April 2020.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Prim. 2018;4:18008.
Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16:30–40.
Nordentoft M, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS ONE. 2013;8:e55176.
Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13:153–60.
Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60:147–56.
De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24:412–24.
Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55:752–60.
Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298:1794–6.
Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72:334–41.
Goodwin RD, Davidson KW, Keyes K. Mental disorders and cardiovascular disease among adults in the United States. J Psychiatr Res. 2009;43:239–46.
WHO report. http://www.euro.who.int/__data/assets/pdf_file/0009/342297/Comorbidity-report_E-web.pdf. Accessed 25 April 2020.
Fries GR, Bauer IE, Scaini G, Valvassori SS, Walss-Bass C, Soares JC, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Disord. 2019. https://doi.org/10.1111/bdi.12876 [Online ahead of print].
Hajek T, Franke K, Kolenic M, Capkova J, Matejka M, Propper L, et al. Brain age in early stages of bipolar disorders or schizophrenia. Schizophr Bull. 2019;45:190–98.
Nenadic I, Dietzek M, Langbein K, Sauer H, Gaser C. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res Neuroimaging. 2017;266:86–89.
Shahab S, Mulsant BH, Levesque ML, Calarco N, Nazeri A, Wheeler AL, et al. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019;44:898–906.
Van Gestel H, Franke K, Petite J, Slaney C, Garnham J, Helmick C, et al. Brain age in bipolar disorders: effects of lithium treatment. Aust N Z J Psychiatry. 2019;53:1179–88.
Squassina A, Pisanu C, Vanni R. Mood disorders, accelerated aging, and inflammation: is the link hidden in telomeres? Cells. 2019;8:52.
Giardini MA, Segatto M, da Silva MS, Nunes VS, Cano MI. Telomere and telomerase biology. Prog Mol Biol Transl Sci. 2014;125:1–40.
Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584–93.
Rode L, Nordestgaard BG, Bojesen SE. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst. 2015;107:djv074.
Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL. Relationship between leukocyte telomere length, telomerase activity, and hippocampal volume in early aging. JAMA Neurol. 2014;71:921–3.
King KS, Kozlitina J, Rosenberg RN, Peshock RM, McColl RW, Garcia CK. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014;71:1247–54.
Staffaroni AM, Tosun D, Lin J, Elahi FM, Casaletto KB, Wynn MJ, et al. Telomere attrition is associated with declines in medial temporal lobe volume and white matter microstructure in functionally independent older adults. Neurobiol Aging. 2018;69:68–75.
Wikgren M, Karlsson T, Soderlund H, Nordin A, Roos G, Nilsson LG, et al. Shorter telomere length is linked to brain atrophy and white matter hyperintensities. Age Ageing. 2014;43:212–7.
Shivakumar V, Kalmady SV, Rajasekaran A, Chhabra H, Anekal AC, Narayanaswamy JC, et al. Telomere length and its association with hippocampal gray matter volume in antipsychotic-naive/free schizophrenia patients. Psychiatry Res Neuroimaging. 2018;282:11–7.
Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, et al. The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78:776–87.
Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, et al. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev. 2016;25:55–69.
Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann NY Acad Sci. 2019;1437:57–67.
Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–58.
Pfau ML, Menard C, Russo SJ. Inflammatory mediators in mood disorders: therapeutic opportunities. Annu Rev Pharm Toxicol. 2018;58:411–28.
Capuron L, Castanon N. Role of inflammation in the development of neuropsychiatric symptom domains: evidence and mechanisms. Curr. Top Behav Neurosci. 2017;31:31–44.
Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2018;25:1301–11.
Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–41.
Haroon E, Miller AH. Inflammation Effects on Glutamate as a Pathway to Neuroprogression in Mood Disorders. Mod Trends Pharmacopsychiatry. 2017;31:37–55.
Coutts F, Palmos AB, Duarte RRR, de Jong S, Lewis CM, Dima D, et al. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology. 2019;44:757–65.
Martinsson L, Wei Y, Xu D, Melas PA, Mathe AA, Schalling M, et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry. 2013;3:e261.
Squassina A, Pisanu C, Congiu D, Caria P, Frau D, Niola P, et al. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur Neuropsychopharmacol. 2016;26:1241–7.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–72.
Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, Lindqvist D, et al. Leukocyte telomere length predicts SSRI response in major depressive disorder: a preliminary report. Mol Neuropsychiatry. 2016;2:88–96.
Manchia M, Paribello P, Arzedi C, Bocchetta A, Caria P, Cocco C, et al. A multidisciplinary approach to mental illness: do inflammation, telomere length and microbiota form a loop? A protocol for a cross-sectional study on the complex relationship between inflammation, telomere length, gut microbiota and psychiatric disorders. BMJ Open. 2020;10:e032513.
Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67 Suppl 6:16–22.
Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96.
Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–7.
Manchia M, Adli M, Akula N, Ardau R, Aubry JM, Backlund L, et al. Assessment of response to lithium maintenance treatment in bipolar disorder: a consortium on lithium genetics (ConLiGen) report. PLoS ONE. 2013;8:e65636.
Scott J, Etain B, Manchia M, Brichant-Petitjean C, Geoffroy PA, Schulze T, et al. An examination of the quality and performance of the Alda scale for classifying lithium response phenotypes. Bipolar Disord. 2019;22:255–65.
First MB, Spitzer RL, Gibbon M, Williams JBW Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute, 2002.
Cantara S, Pisu M, Frau DV, Caria P, Dettori T, Capezzone M, et al. Telomere abnormalities and chromosome fragility in patients affected by familial papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E1327–31.
Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166 e17–28.
Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8.
Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann NY Acad Sci. 1982;389:406–18.
Faugere M, Micoulaud-Franchi JA, Faget-Agius C, Lancon C, Cermolacce M, Richieri R. High C-reactive protein levels are associated with depressive symptoms in schizophrenia. J Affect Disord. 2018;225:671–75.
Fond G, Lancon C, Auquier P, Boyer L. C-reactive protein as a peripheral biomarker in schizophrenia. an updated systematic review. Front Psychiatry. 2018;9:392.
Sicras-Mainar A, Rejas-Gutierrez J, Navarro-Artieda R, Blanca-Tamayo M. C-reactive protein as a marker of cardiovascular disease in patients with a schizophrenia spectrum disorder treated in routine medical practice. Eur Psychiatry. 2013;28:161–7.
Wysokinski A, Margulska A, Strzelecki D, Kloszewska I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord J Psychiatry. 2015;69:346–53.
Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–64.
Rao S, Kota LN, Li Z, Yao Y, Tang J, Mao C, et al. Accelerated leukocyte telomere erosion in schizophrenia: evidence from the present study and a meta-analysis. J Psychiatr Res. 2016;79:50–6.
Russo P, Prinzi G, Proietti S, Lamonaca P, Frustaci A, Boccia S, et al. Shorter telomere length in schizophrenia: evidence from a real-world population and meta-analysis of most recent literature. Schizophr Res. 2018;202:37–45.
Higgins-Chen AT, Boks MP, Vinkers CH, Kahn RS, Levine ME. Schizophrenia and epigenetic aging biomarkers: increased mortality, reduced cancer risk, and unique clozapine effects. Biol Psychiatry. 2020;88:224–35.
Lu AT, Seeboth A, Tsai PC, Sun D, Quach A, Reiner AP, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY). 2019;11:5895–923.
Li Z, Hu M, Zong X, He Y, Wang D, Dai L, et al. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naive schizophrenia. Sci Rep. 2015;5:18553.
Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–7.
Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull. 2018;44:398–408.
Osler M, Bendix L, Rask L, Rod NH. Stressful life events and leucocyte telomere length: do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav Immun. 2016;58:248–53.
Ayari F, Ben Chaaben A, Ben Ammar H, Nefzi R, Ouni N, Mihoub O, et al. Association of high-sensitivity C-reactive protein with susceptibility to Schizophrenia in Tunisian population. Encephale. 2020;46:241–7.
Glaus J, von Kanel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 2018;48:961–73.
Leung BMY, Nwoke C. Association between C-reactive protein and mood disorder in a representative sample of the Canadian population: analysis of CHMS data 2013-4. Can J Public Health. 2020. https://doi.org/10.17269/s41997-020-00297-3 [Online ahead of print].
Marshe VS, Pira S, Mantere O, Bosche B, Looper KJ, Herrmann N, et al. C-reactive protein and cardiovascular risk in bipolar disorder patients: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:442–51.
Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214:11–9.
Ruland T, Chan MK, Stocki P, Grosse L, Rothermundt M, Cooper JD, et al. Molecular serum signature of treatment resistant depression. Psychopharmacol (Berl). 2016;233:3051–9.
Strawbridge R, Hodsoll J, Powell TR, Hotopf M, Hatch SL, Breen G, et al. Inflammatory profiles of severe treatment-resistant depression. J Affect Disord. 2019;246:42–51.
Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38:377–85.
Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–9.
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41.
Pinna M, Manchia M, Oppo R, Scano F, Pillai G, Loche AP, et al. Clinical and biological predictors of response to electroconvulsive therapy (ECT): a review. Neurosci Lett. 2018;669:32–42.
Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19:183–8.
Yrondi A, Sporer M, Peran P, Schmitt L, Arbus C, Sauvaget A. Electroconvulsive therapy, depression, the immune system and inflammation: a systematic review. Brain Stimul. 2018;11:29–51.
Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79:24–38.
Chang HH, Chen PS. Inflammatory biomarkers for mood disorders–a brief narrative review. Curr Pharm Des. 2020;26:236–43.
Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91.
Kohler O, Krogh J, Mors O, Benros ME. Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol. 2016;14:732–42.
Miller AH, Raison CL. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA Psychiatry. 2015;72:527–8.
Muller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44:973–82.
Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BT, et al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18:89–101.
Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17:343–63.
Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: a meta-analysis. J Affect Disord. 2016;191:237–47.
Lin PY, Huang YC, Hung CF. Shortened telomere length in patients with depression: a meta-analytic study. J Psychiatr Res. 2016;76:84–93.
Pisanu C, Tsermpini EE, Skokou M, Kordou Z, Gourzis P, Assimakopoulos K, et al. Leukocyte telomere length is reduced in patients with major depressive disorder. Drug Dev Res. 2020;81:268–73.
Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X, et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci. 2011;31:12258–69.
Powell TR, Dima D, Frangou S, Breen G. Telomere length and bipolar disorder. Neuropsychopharmacology. 2018;43:454.
Pisanu C, Congiu D, Manchia M, Caria P, Cocco C, Dettori T, et al. Differences in telomere length between patients with bipolar disorder and controls are influenced by lithium treatment. Pharmacogenomics. 2020;21:533–40.
Jose SS, Bendickova K, Kepak T, Krenova Z, Fric J. Chronic inflammation in immune aging: role of pattern recognition receptor crosstalk with the telomere complex? Front Immunol. 2017;8:1078.
Boeck C, Salinas-Manrique J, Calzia E, Radermacher P, von Arnim CAF, Dietrich DE, et al. Targeting the association between telomere length and immuno-cellular bioenergetics in female patients with major depressive disorder. Sci Rep. 2018;8:9419.
Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50.
Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192.
Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–38.
Author information
Authors and Affiliations
Contributions
AS conceived and designed the work, acquired the data, contributed interpreting the results, drafted and revised the manuscript, approved the final version; MM performed and coordinated the recruitment of patients, participated conceiving the study and drafting the manuscript; CP run part of the experiments, drafted the methods and result section, contributed analyzing and interpreting the findings; DC, performed part of the TL experiments and coordinated the activity of the laboratory of pharmacogenomics; AM, performed part of the experiments on telomeres; RA, CA, AB, EC, MG, MAM, AM, PP, FP, GS, contributed recruiting the patients and controls; PC, TD, DVF performed the Q-FISH experiments; MN, RR, VS contributed conceiving the study and interpreting the results; CC, EM, BN performed the ELISA experiments; GLF coordinated the ELISA experiments and contributed interpreting the findings; MDZ and CC coordinated the recruitment of patients and controls at the Unit of Clinical Pharmacology, and contributed designing the study; RV contributed conceiving the study, interpreting findings and finalizing the manuscript; BC coordinated the clinical activity of the Unit of Psychiatry and contributed interpreting the findings and finalizing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Squassina, A., Manchia, M., Pisanu, C. et al. Telomere attrition and inflammatory load in severe psychiatric disorders and in response to psychotropic medications. Neuropsychopharmacol. 45, 2229–2238 (2020). https://doi.org/10.1038/s41386-020-00844-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-00844-z
This article is cited by
-
Premature aging in serious mental illness
Neuropsychopharmacology (2026)
-
Exploring the association between depression and telomere length: A systematic review and meta-analysis
Scientific Reports (2025)
-
Association of the newly proposed dietary index for gut microbiota and depression: the mediation effect of phenotypic age and body mass index
European Archives of Psychiatry and Clinical Neuroscience (2025)
-
Dissecting the genetic overlap between severe mental disorders and markers of cellular aging: Identification of pleiotropic genes and druggable targets
Neuropsychopharmacology (2024)
-
Immunophenotypes in psychosis: is it a premature inflamm-aging disorder?
Molecular Psychiatry (2024)