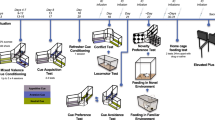
Abstract
Approach-avoidance conflict is induced when an organism encounters a stimulus that carries both positive and negative attributes. Accumulating evidence implicates the ventral hippocampus (VH) in the detection and resolution of approach-avoidance conflict, largely on the basis of maze-based tasks assaying innate and conditioned responses to situations of conflict. However, its role in discrete trial approach-avoidance decision-making has yet to be elucidated. In this study, we designed a novel cued operant conflict decision-making task in which rats were required to choose and respond for a low reward option or high reward option paired with varying shock intensities on a differential reinforcement of low rates of responding schedule. Post training, the VH was chemogenetically inhibited while animals performed the task with the usual outcomes delivered, and with the presentation of cues associated with the reward vs. conflict options only (extinction condition). We found that VH inhibition led to an avoidance of the conflict option and longer latency to choose this option when decision-making was being made on the basis of cues alone with no outcomes. Consistent with these findings, VH-inhibited animals spent more time in the central component of the elevated plus maze (EPM), indicating a potential deficit in decision-making under innate forms of approach-avoidance conflict. Taken together, these findings implicate the VH in cue-driven approach-avoidance decisions in the face of motivational conflict.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–56.
Bailey MR, Simpson EH, Balsam PD. Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol Learn Mem. 2016;133:233–56.
Smith S, Guthrie ER. General psychology in terms of behavior. New York, NY: D. Appleton & Co.; 1921.
Brown JS. Generalized approach and avoidance responses in relation to conflict behavior. Doctoral dissertation. Yale University, 1940.
Brown JS. The generalization of approach responses as a function of stimulus intensity and strength of motivation. J Comp Psychol. 1942;33:209.
Brown JS. Gradients of approach and avoidance responses and their relation to level of motivation. J Comp Physiol Psychol. 1948;41:450.
Miller NE, Murray EJ. Displacement and conflict; learnable drive as a basis for the steeper gradient of avoidance than of approach. J Exp Psychol. 1952;43:227–31.
Nguyen D, Schumacher A, Erb S, Ito R. Aberrant approach-avoidance conflict resolution following repeated cocaine pre-exposure. Psychopharmacology. 2015;232:3573–83.
Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp. 2015;36:449–62.
Fricke K, Vogel S. How interindividual differences shape approach-avoidance behavior: relating self-report and diagnostic measures of interindividual differences to behavioral measurements of approach and avoidance. Neurosci Biobehav Rev. 2020;111:30–56.
Bannerman DM, Rawlins JNP, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83.
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 2018;97:670–83.
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66.
Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38.
Çavdaroğlu B, Toy J, Schumacher A, Carvalho G, Patel M, Ito R. Ventral hippocampus inactivation enhances the extinction of active avoidance responses in the presence of safety signals but leaves discrete trial operant active avoidance performance intact. Hippocampus. 2020. https://doi.org/10.1002/hipo.23202.
Schumacher A, Villaruel FR, Ussling A, Riaz S, Lee ACH, Ito R. Ventral hippocampal CA1 and CA3 differentially mediate learned approach-avoidance conflict processing. Curr Biol. 2018;28:1318–24.e4.
Schumacher A, Vlassov E, Ito R. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 2016;26:530–42.
Ito R, Lee ACH. The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav Brain Res. 2016;313:345–57.
Bach DR, Guitart-Masip M, Packard PA, Miró J, Falip M, Fuentemilla L, et al. Human hippocampus arbitrates approach-avoidance conflict. Curr Biol. 2014;24:541–7.
O’Neil EB, Newsome RN, Li IHN, Thavabalasingam S, Ito R, Lee ACH. Examining the role of the human hippocampus in approach-avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J Neurosci. 2015;35:15039–49.
Wallis CU, Cockcroft GJ, Cardinal RN, Roberts AC, Clarke HF. Hippocampal interaction with area 25, but not area 32, regulates marmoset approach–avoidance behavior. Cereb Cortex. 2019. https://doi.org/10.1093/cercor/bhz015.
Talmi D, Pine A. How costs influence decision values for mixed outcomes. Front Neurosci. 2012;6:1–21.
Park SQ, Kahnt T, Rieskamp J, Heekeren HR. Neurobiology of value integration: when value impacts valuation. J Neurosci. 2011;31:9307–14.
Cheung THC, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci. 2005. https://doi.org/10.1186/1471-2202-6-36.
Freestone DM, Church RM. Optimal timing. Curr Opin Behav Sci. 2016;8:276–81.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–75.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Team R. Core. R: A language and environment for statistical computing. Ind Commer Train. 2019. https://doi.org/10.1108/eb003648.
Bates D, Maechler M, Bolker B, Walker S. Package“lme4”. J Stat Softw. 2015. https://doi.org/10.18637/jss.v067.i01.
Cavdaroglu B, Zeki M, Balci F. Time-based reward maximization. Philos Trans R Soc B Biol Sci. 2014;369:20120461–20120461.
Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001;63:1293–313.
Orsini CA, Trotta RT, Bizon JL, Setlow B. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J Neurosci. 2015;35:1368–379.
Orsini CA, Hernandez CM, Singhal S, Kelly KB, Frazier CJ, Bizon JL, et al. Optogenetic inhibition reveals distinct roles for basolateral amygdala activity at discrete time points during risky decision making. J Neurosci. 2017;37:11537–48.
Vento PJ, Burnham NW, Rowley CS, Jhou TC. Learning from one’s mistakes: a dual role for the rostromedial tegmental nucleus in the encoding and expression of punished reward seeking. Biol Psychiatry. 2017;81:1041–9.
Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–43.
van den Bos R, Lasthuis W, den Heijer E, van der Harst J, Spruijt B. Toward a rodent model of the Iowa gambling task. Behav Res Methods. 2006;38:470–8.
Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–70.
Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37.
Winstanley CA, Floresco SB. Deciphering decision making: variation in animal models of effort- and uncertainty-based choice reveals distinct neural circuitries underlying core cognitive processes. J Neurosci. 2016;36:12069–79.
Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–17.
Oberrauch S, Sigrist H, Sautter E, Gerster S, Bach DR, Pryce CR. Establishing operant conflict tests for the translational study of anxiety in mice. Psychopharmacology. 2019;236:2527–41.
Abela AR, Dougherty SD, Fagen ED, Hill CJR, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex. 2013;23:1396–409.
Freestone DM, Balcı F, Simen P, Church RM. Optimal response rates in humans and rats. J Exp Psychol Anim Learn Cogn. 2015;41:39–51.
Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JNP. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901.
Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JNP. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–88.
Sutherland RJ, McDonald RJ, Hill CR, Rudy JW. Damage to the hippocampal formation in rats selectively impairs the ability to learn cue relationships. Behav Neural Biol. 1989;52:331–56.
Ito R, Everitt BJ, Robbins TW. The hippocampus and appetitive Pavlovian conditioning: effects of excitotoxic hippocampal lesions on conditioned locomotor activity and autoshaping. Hippocampus. 2005;15:713–21.
Riaz S, Schumacher A, Sivagurunathan S, Van Der Meer M, Ito R. Ventral, but not dorsal, hippocampus inactivation impairs reward memory expression and retrieval in contexts defined by proximal cues. Hippocampus. 2017;27:822–36.
Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron 2014;81:267–79.
Bradfield LA, Dezfouli A, Van Holstein M, Chieng B, Balleine BW. Medial orbitofrontal cortex mediates outcome retrieval in partially observable task situations. Neuron. 2015;88:1268–80.
Wikenheiser AM, Schoenbaum G. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci. 2016;17:513–23.
Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron. 2017;95:1197–207.
Jarrard LE, Isaacson RL. Hippocampal ablation in rats: effects of intertrial interval. Nature. 1965;207:109–10.
Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75.
De Martino B, Fleming SM, Garrett N, Dolan RJ. Confidence in value-based choice. Nat Neurosci. 2013;16:105–10.
Boguszewski P, Zagrodzka J. Emotional changes related to age in rats—a behavioral analysis. Behav Brain Res. 2002;133:323–32.
Yeates DCM, Ussling A, Lee ACH, Ito R. Double dissociation of learned approach–avoidance conflict processing and spatial pattern separation along the dorsoventral axis of the dentate gyrus. Hippocampus. 2019. https://doi.org/10.1002/hipo.23182.
Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112.
Bannerman D, Grubb M, Deacon RM, Yee B, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213.
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002. https://doi.org/10.1073/pnas.152112399.
Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–84.
Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19.
Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–68.
Engin E, Smith KS, Gao Y, Nagy D, Foster RA, Tsvetkov E, et al. Modulation of anxiety and fear via distinct intrahippocampal circuits. Elife. 2016;5:e14120.
Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA. 2009. https://doi.org/10.1073/pnas.0812608106.
Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Selective information routing by ventral hippocampal CA1 projection neurons. Science. 2015;348:560–3.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Acad Press. 1998. p. 1–474.
Acknowledgements
We are grateful to Dr. Maithe Arruda Carvalho for providing the training and use of her Nikon-Ni U fluorescent microscope.
Author information
Authors and Affiliations
Contributions
Conceptualization, BC, RI; methodology, BC, RI; data collection, BC, SR, EY; data analysis, BC; visualization, BC, RI; writing, review, and editing, BC, ACHL, RI.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Çavdaroğlu, B., Riaz, S., Yeung, E.H.L. et al. The ventral hippocampus is necessary for cue-elicited, but not outcome driven approach-avoidance conflict decisions: a novel operant choice decision-making task. Neuropsychopharmacol. 46, 632–642 (2021). https://doi.org/10.1038/s41386-020-00898-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-00898-z
This article is cited by
-
Blocking μ-opioid receptors attenuates reinstatement of responding to an alcohol-predictive conditioned stimulus through actions in the ventral hippocampus
Neuropsychopharmacology (2023)
-
Two opposing hippocampus to prefrontal cortex pathways for the control of approach and avoidance behaviour
Nature Communications (2022)
-
Parallel ventral hippocampus-lateral septum pathways differentially regulate approach-avoidance conflict
Nature Communications (2022)