Abstract
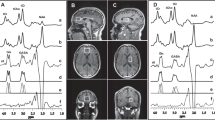
Youth at clinical high risk (CHR) are a unique population enriched for precursors of major psychiatric disorders, especially schizophrenia (SCZ). Recent neuroimaging findings point to abnormalities in the thalamus of patients with SCZ, including chronic and early course patients, as well as in CHR individuals relative to healthy comparison groups, thus suggesting that thalamic dysfunctions are present even before illness onset. Furthermore, modeling data indicate that alteration between excitatory and inhibitory control, as reflected by alteration in GABAergic and glutamatergic balance (i.e., GABA/Glu), may underlie thalamic deficits linked to the risk and development of psychosis. There is, however, a lack of in vivo evidence of GABA/Glu thalamic abnormalities in the CHR state. Magnetic resonance spectroscopic imaging (MRSI) 7 Tesla (7 T) provides enhanced resolution to quantify GABA and Glu levels in the thalamus of CHR individuals. In this study, we performed 7 T MRSI in 15 CHR and 20 healthy control (HC) participants. We found that GABA/Glu was significantly reduced in the right medial anterior and right medial posterior thalamus of CHR relative to HC groups. The GABA/Glu reduction was negatively correlated with general symptoms in the right medial anterior thalamus, as well as with disorganization symptoms in the right medial posterior thalamus. Altogether, these findings indicate that GABA/Glu abnormalities are present in the thalamus before the onset of full-blown psychosis and are associated with symptom severity, thus providing putative molecular and neuronal targets for early interventions in youth at CHR.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, Watts AKS, et al. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151:148–53.
Cooper D, Barker V, Radua J, Fusar-Poli P, Lawrie SM. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res. 2014;221:69–77.
Harrisberger F, Buechler R, Smieskova R, Lenz C, Walter A, Egloff L, et al. Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. NPJ Schizophr. 2016;2:1–6.
Cho KIK, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun J-Y, et al. Altered thalamo-cortical white matter connectivity: probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 2016;42:723–31.
Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72:882–91.
Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9:1–9.
Steullet P. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res. 2019. https://doi.org/10.1016/j.schres.2019.05.027.
Cai H-L, Zhu R-H, Zhang X-H, Hu L, Yang W, Ye H-S. Elevated plasma γ-aminobutyrate/glutamate ratio and responses to risperidone antipsychotic treatment in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1273–8.
Deutch AY, Roth RH. Pharmacology and biochemistry of synaptic transmission: classical transmitters. In: Byrne JH, Heidelberger R, Waxham MN, editors. From molecules to networks. An introduction to cellular and molecular neuroscience. Elsevier; 2014. p. 245–78.
Waagepetersen HS, Sonnerwald U, Schousboe A. Glutamine, glutamate and GABA: metabolic aspects. In: Lajtha A, Oja SS, Schousboe A, Saransaari P, editors. Handbook of neurochemistry and molecular neurobiology. Springer; 2007. p. 1–21.
Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia—a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503.
Fusar-Poli P, Stone J, Broome M, Valli I, Mechelli A, McLean M, et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry. 2011;68:881–90.
Pan J, Duckrow R, Spencer D, Avdievich N, Hetherington H. Selective homonuclear polarization transfer for spectroscopic imaging of GABA at 7T. Magn Reson Med. 2013;69:310–16.
Cudalbu C, Mlynárik V, Gruetter R. Handling macromolecule signals in the quantification of the neurochemical profile. J Alzheimers Dis. 2012;31:S101–15.
Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–85.
Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RA, et al. Comparison of single voxel brain MRS AT 3 T and 7 T using 32-channel head coils. Magn Reson Imaging. 2015;33:1013–8.
Andreychenko A, Boer VO, Arteaga de Castro CS, Luijten PR, Klomp DW. Efficient spectral editing at 7 T: GABA detection with MEGA‐sLASER. Magn Reson Med. 2012;68:1018–25.
Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15.
Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med. 2012;68:1007–17.
Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63:9–19.
Pan J, Avdievich N, Hetherington H. J‐refocused coherence transfer spectroscopic imaging at 7 T in human brain. Magn Reson Med. 2010;64:1237–46.
Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49:1271–81.
Hetherington H, Kuzniecky R, Vives K, Devinsky O, Pacia S, Luciano D, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology 2007;69:2256–65.
Twieg D, Meyerhoff D, Hubesch B, Roth K, Sappey‐Marinier D, Boska MD, et al. Phosphorus‐31 magnetic resonance spectroscopy in humans by spectroscopic imaging: localized spectroscopy and metabolite imaging. Magn Reson Med. 1989;12:291–305.
Smith S, Levante T, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson Ser A. 1994;106:75–105.
Öz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014;270:658–79.
Murray JD, Anticevic A. Toward understanding thalamocortical dysfunction in schizophrenia through computational models of neural circuit dynamics. Schizophr Res. 2017;180:70–7.
Wenneberg C, Glenthoj BY, Hjorthoj C, Buchardt Zingenberg FJ, Glenthoj LB, Rostrup E, et al. Cerebral glutamate and GABA levels in high-risk of psychosis states: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Res. 2020;215:38–48.
Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–9.
Valli I, Stone J, Mechelli A, Bhattacharyya S, Raffin M, Allen P, et al. Altered medial temporal activation related to local glutamate levels in subjects with prodromal signs of psychosis. Biol Psychiatry. 2011;69:97–9.
Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, et al. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology. 2014;39:2891–9.
Connett RJ. Analysis of metabolic control: new insights using scaled creatine kinase model. Am J Physiol Regul Integr Comp Physiol. 1988;254:R949–59.
Öngür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–8.
Tibbo PG, Bernier D, Hanstock CC, Seres P, Lakusta B, Purdon SE. 3‐T proton magnetic spectroscopy in unmedicated first episode psychosis: a focus on creatine. Magn Reson Med. 2013;69:613–20.
Buchmann A, Dentico D, Peterson MJ, Riedner BA, Sarasso S, Massimini M, et al. Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage 2014;102:540–47.
Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 2018;83:509–17.
Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–25.
Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–9.
Sheffield JM, Huang AS, Rogers BP, Giraldo-Chica M, Landman BA, Blackford JU, et al. Thalamocortical anatomical connectivity in schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2020;46:1062–71.
Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56.
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57.
Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92.
Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–48.
Kaskie RE, Graziano B, Ferrarelli F. Topographic deficits in sleep spindle density and duration point to frontal thalamo-cortical dysfunctions in first-episode psychosis. J Psychiatr Res. 2019;113:39–44.
Li T, Wang Q, Zhang J, Rolls ET, Yang W, Palaniyappan L, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–48.
Corcoran C, Kimhy D, Parrilla-Escobar M, Cressman V, Stanford A, Thompson J, et al. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41:251.
Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–9.
Reddy-Thootkur M, Kraguljac NV, Lahti AC. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders–a systematic review of magnetic resonance spectroscopy studies. Schizophr Res. 2020. https://doi.org/10.1016/j.schres.2020.02.001.
Takahashi T, Tsugawa S, Nakajima S, Plitman E, Chakravarty MM, Masuda F, et al. Thalamic and striato-pallidal volumes in schizophrenia patients and individuals at risk for psychosis: a multi-atlas segmentation study. Schizophr Res. 2020. https://doi.org/10.1016/j.schres.2020.04.016.
Bojesen KB, Broberg BV, Fagerlund B, Jessen K, Thomas MB, Sigvard A, et al. Associations between cognitive function and levels of glutamatergic metabolites and GABA in antipsychotic-naïve patients with schizophrenia or psychosis. Biol Psychiatry. 2020. https://doi.org/10.1016/j.biopsych.2020.06.027.
Vukadinovic Z, Rosenzweig I. Abnormalities in thalamic neurophysiology in schizophrenia: could psychosis be a result of potassium channel dysfunction? Neurosci Biobehav Rev. 2012;36:960–8.
Seeman P, Guan H-C, Van, Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature 1993;365:441–5.
Danos P, Baumann B, Krämer A, Bernstein H-G, Stauch R, Krell D, et al. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res. 2003;60:141–55.
Author information
Authors and Affiliations
Contributions
GMQ and HPH contributed to the acquisition, analysis and interpretation of data, drafting and revising the work, and approving the final version to be published. AM and VEY contributed to the analysis and interpretation of data, drafting and revising the work, and approving the final version to be published. FF contributed to the conception and design of the work, the analysis and interpretation of data, drafting and revising the work, and approving the final version to be published.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Quiñones, G.M., Mayeli, A., Yushmanov, V.E. et al. Reduced GABA/glutamate in the thalamus of individuals at clinical high risk for psychosis. Neuropsychopharmacol. 46, 1133–1139 (2021). https://doi.org/10.1038/s41386-020-00920-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-00920-4
This article is cited by
-
Leveraging ultra-high field (7T) MRI in psychiatric research
Neuropsychopharmacology (2025)
-
Multimodal evidence of mediodorsal thalamus-prefrontal circuit dysfunctions in clinical high-risk for psychosis: findings from a combined 7T fMRI, MRSI and sleep Hd-EEG study
Molecular Psychiatry (2025)
-
[1H-13C]-NMR-Based Metabolic Kinetics Reveals Brain Neurochemical Alterations in Mice After Retinal Ischemia–Reperfusion Injury
Molecular Neurobiology (2025)
-
A phylogenetically-conserved axis of thalamocortical connectivity in the human brain
Nature Communications (2023)