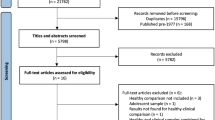
Abstract
Early interventions to improve resilience require the identification of objective risk biomarkers for PTSD symptom development. Although altered hippocampal and amygdala volumes are consistently observed in PTSD, it remains currently unknown whether they represent a predisposing vulnerability factor for PTSD symptom development or an acquired consequence of trauma exposure and/or the disorder. We conducted a longitudinal, prospective study in 210 police recruits at high risk for trauma exposure (56 females(26.7%); mean[SD] age = 24.02[5.19]). Structural MRI scans and trauma-related symptom severity were assessed at pre-trauma baseline and at 16-month follow-up. Between assessments, police recruits were exposed to various potentially traumatic events during their police training. Police recruits reported a significant increase in police-related trauma exposure and stress-related symptoms between assessments. Smaller hippocampal left dentate gyrus (DG) volumes at baseline predicted increase in self-reported PTSD symptoms (B[SE] = −0.21[0.08], p = 0.011), stress symptoms (B[SE] = −0.16[0.07], p = 0.024) and negative affect (B[SE] = −0.21[0.07], p = 0.005) upon trauma exposure. Amount of police-related trauma exposure between assessments was positively associated with an increase in left basal amygdala nucleus volume (B[SE] = 0.11[0.05], p = 0.026). Taken together, smaller DG-volumes pre-trauma may represent a predisposing neurobiological vulnerability factor for development of trauma-related symptoms. On the other hand, amount of trauma exposure between assessments was positively associated with increased amygdala basal nucleus volume, suggesting acquired neural effects. These findings suggest that preventive interventions for PTSD aimed at improving resilience could be targeted at increasing DG-volume and potentially its functioning.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Goldstein R, Smith S, Chou S, Saha T, Jung J, Zhang H, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51:1137–48.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87.
Koch SBJ, Morey RA, Roelofs K. The role of the dentate gyrus in stress-related disorders. Mol Psychiatry. 2019;25:1361–63.
American Psychiatric Association. DSM-5, diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA, US: American Psychiatric Publishing Inc.; 2013.
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30.
Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am J Psychiatry. 2018;175:989–98.
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83:244–53.
Nelson MD, Tumpap AM. Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: a meta-analytic study. CNS Spectr. 2017;22:363–72.
Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–78.
Gilbertson M, Shenton M, Ciszewski A. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;i:1242–7.
van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med. 2015;45:2737–46.
Lindgren L, Bergdahl J, Nyberg L. Longitudinal evidence for smaller hippocampus volume as a vulnerability factor for perceived stress. 2016. https://doi.org/10.1093/cercor/bhw154.
Quidé Y, Andersson F, Dufour-Rainfray D, Descriaud C, Brizard B, Gissot V, et al. Smaller hippocampal volume following sexual assault in women is associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2018;138:312–24.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–50.
Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47.
Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res Neuroimaging. 2015;233:436–42.
Kleim B, Ehlers A, Glucksman E. Early predictors of chronic post-traumatic stress disorder in assault survivors. Psychol Med. 2007;37:1457–67.
Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–20.
Leal SL, Yassa MA. Integrating new findings and examining clinical applications of pattern separation. Nat Neurosci. 2018;21:163–73.
Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M. Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. J Psychiatr Res. 2017;95:247–52.
Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303.
Luo Y, Liu Y, Qin Y, Zhang X, Ma T, Wu W, et al. The atrophy and laterality of the hippocampal subfields in parents with or without posttraumatic stress disorder who lost their only child in China. Neurol Sci. 2017;38:1241–7.
Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, et al. Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety. 2018;35:1018–29.
Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, Ranganath C. CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nat Commun. 2018;9:1–8.
Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15:244–51.
Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31.
Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–604.
Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress. 2017;1:2470547017724069.
Morey RA, Clarke EK, Haswell CC, Phillips RD, Clausen AN, Mufford MS, et al. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2020;5:281–90.
Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: a review and statistical evaluation. Clin Psychol Rev. 2018;63:41–55.
Koch SBJ, Klumpers F, Zhang W, Hashemi MM, Kaldewaij R, van Ast VA, et al. The role of automatic defensive responses in the development of posttraumatic stress symptoms in police recruits: protocol of a prospective study. Eur J Psychotraumatol. 2017;8:1412226.
Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov. 2013.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90.
Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatr Pract. 2004;10:307–13.
Carlier I, Gersons B. Development of a scale for traumatic incidents in police work. Psychiatr Fenn. 1992;23:59–70.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37.
Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th Edn. Boston: Allyn and Bacon; 2001.
George D, Mallery M. SPSS for windows step by step: a simple guide and reference, 17.0 update. 10a ed. Boston: Pearson; 2010.
Hayes AF, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods. 2007;39:709–22.
Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The clinician-administered PTSD scale for DSM-5 (CAPS-5). Interview available from the National Center for PTSD at www.ptsd.va.gov. 2013.
Peter-Hagene LC, Ullman SE. Sexual assault-characteristics effects on PTSD and psychosocial mediators: a cluster-analysis approach to sexual assault types. Psychol Trauma. 2015;7:162–70.
Brancu M, Mann-Wrobel M, Beckham JC, Wagner HR, Elliott A, Robbins AT, et al. Subthreshold posttraumatic stress disorder: a meta-analytic review of DSM–IV prevalence and a proposed DSM–5 approach to measurement. Psychol Trauma Theory, Res Pr Policy. 2016;8:222–32.
Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102.
Nuninga JO, Mandl RCW, Boks MP, Bakker S, Somers M, Heringa SM, et al. Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Molecular Psychiatry. 2020;25;1559–68.
Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry. 2015;5:e700.
Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–72.
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–22.
Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73.
Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA. 2008;105:5573–8.
Liu H, Petukhova MV, Sampson NA, Aguilar-Gaxiola S, Alonso J, Andrade LH, et al. Association of DSM-IV posttraumatic stress disorder with traumatic experience type and history in the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74:270.
van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–71.
Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204.
Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur J Psychotraumatol. 2017;8;1351204. https://doi.org/10.1080/20008198.2017.1351204.
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–77.
Haukvik UK, Westlye LT, Mørch-Johnsen L, Jørgensen KN, Lange EH, Dale AM, et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77:581–8.
Shu X-JJ, Xue L, Liu W, Chen F-YY, Zhu C, Sun X-HH, et al. More vulnerability of left than right hippocampal damage in right-handed patients with post-traumatic stress disorder. Psychiatry Res. 2013;212:237–44.
Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–25.
Villarreal G, Petropoulos H, Hamilton DA, Rowland LM, Horan WP, Griego JA, et al. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: preliminary results. Can J PsychiatryRevue Can Psychiatr. 2002;47:666–70.
Lindauer RJL, Vlieger EJ, Jalink M, Olff M, Carlier IVE, Majoie CBLM, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–63.
van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, et al. Increased activation of the fear neurocircuitry in children exposed to violence. Depress Anxiety. 2020. https://doi.org/10.1002/da.22994.
Acknowledgements
We thank all participants for their willingness to participate in this study. The authors thank Annika Smit and other personnel of the Dutch Police Academy for their valuable help with recruiting participants and facilitating our study. We gratefully acknowledge contributions of the Ingrid Kerstens, Tiele Döpp, Naomi de Valk, Leonore Bovy and Lisanne Nuijen in participant recruitment and data acquisition.
Author information
Authors and Affiliations
Contributions
All authors contributed to designing the study. RK, WZ & MMH acquired the data and SBJK analyzed the data. All authors contributed to interpreting the results and writing the manuscript. All authors approved the final version of the manuscript before submission.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Koch, S.B.J., van Ast, V.A., Kaldewaij, R. et al. Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms. Neuropsychopharmacol. 46, 1283–1292 (2021). https://doi.org/10.1038/s41386-020-00947-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-00947-7
This article is cited by
-
Neuroimaging of posttraumatic stress disorder in adults and youth: progress over the last decade on three leading questions of the field
Molecular Psychiatry (2024)
-
Longitudinal volumetric evaluation of hippocampus and amygdala subregions in recent trauma survivors
Molecular Psychiatry (2023)
-
Sex-dependent risk factors for PTSD: a prospective structural MRI study
Neuropsychopharmacology (2022)
-
Structural covariance of the ventral visual stream predicts posttraumatic intrusion and nightmare symptoms: a multivariate data fusion analysis
Translational Psychiatry (2022)
-
Neural contributors to trauma resilience: a review of longitudinal neuroimaging studies
Translational Psychiatry (2021)