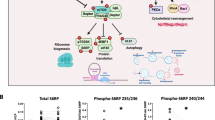
Abstract
Abnormal neurotransmission is central to schizophrenia (SZ). Alterations across multiple neurotransmitter systems in SZ suggest that this illness may be associated with dysregulation of core intracellular processes such as signaling pathways that underlie the regulation and integration of these systems. The AKT-mTOR signaling cascade has been implicated in SZ by gene association, postmortem brain and animal studies. AKT and mTOR are serine/threonine kinases which play important roles in cell growth, proliferation, survival, and differentiation. Both AKT and mTOR require phosphorylation at specific sites for their complete activation. mTOR forms two functionally distinct multiprotein complexes, mTOR Complex 1 (mTORC1) and Complex 2 (mTORC2). mTORC1 mediates ribosome biogenesis, protein translation, and autophagy, whereas mTORC2 contributes to actin dynamics. Altered protein synthesis and actin dynamics can lead to an abnormal neuronal morphology resulting in deficits in learning and memory. Currently, there is a lack of direct evidence to support the hypothesis of disrupted mTOR signaling in SZ, and we have addressed this by characterizing this signaling pathway in SZ brain. We found a reduction in AKT and mTOR protein expression and/or phosphorylation state in dorsolateral prefrontal cortex (DLPFC) from 22 pairs of SZ and matched comparison subjects. We also found reduced protein expression of GβL, a subunit protein common to both mTOR complexes. We further investigated mTOR complex-specific subunit composition and phosphorylation state, and found abnormal mTOR expression in both complexes in SZ DLPFC. These findings provide evidence that proteins associated with the AKT-mTOR signaling cascade are downregulated in SZ DLPFC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
Lang UE, Puls I, Müller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20:687–702.
Meador-Woodruff JH. Novel D2-like dopamine receptors in schizophrenic brain. In: Search for the causes of schizophrenia. Heidelberg: Steinkopff; 1999. p. 251–60.
Joyce J, Meador-Woodruff JH. Linking the family of D2 receptors to neuronal circuits in human brain: insights into schizophrenia. Neuropsychopharmacology. 1997;16:375–84.
Gao W. Dopaminergic and glutamatergic dysfunctions in the neuropathophysiology of schizophrenia. In Kudo E, Fujii Y (eds), Dopamine: functions, regulation and health effects. New York: Nova Science Publishers; 2011. p. 167–194.
McCullumsmith RE, Hammond J, Funk A, Meador-Woodruff JH. Recent advances in targeting the ionotropic glutamate receptors in treating schizophrenia. Curr Pharm Biotechnol. 2012;13:1535–42.
Rubio MD, Drummond JB, Meador-Woodruff JH. Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther. 2012;20:1–18.
McGuire JL, Depasquale EA, Funk AJ, O’Donnovan SM, Hasselfeld K, Marwaha S, et al. Abnormalities of signal transduction networks in chronic schizophrenia. NPJ Schizophr. 2017;3:30.
Funk AJ, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012;37:896–905.
Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci. 2012;5:33.
Zheng W, Wang H, Zeng Z, Lin J, Little PJ, Srivastava LK, et al. The possible role of the Akt signaling pathway in schizophrenia. Brain Res. 2012;1470:145–58.
Kitagishi Y, Kobayashi M, Kikuta K, Matsuda S. Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress Res Treat. 2012;2012:752563.
Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA. AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife. 2017;6. https://doi.org/10.7554/eLife.30640.
Radwanska K, Medvedev NI, Pereira GS, Engmann O, Thiede N, Moraes MFD, et al. Mechanism for long-term memory formation when synaptic strengthening is impaired. Proc Natl Acad Sci USA. 2011;108:18471–5.
Liao Y, Hung M-C. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42.
Howell KR, Floyd K, Law AJ. PKBγ/AKT3 loss-of-function causes learning and memory deficits and deregulation of AKT/mTORC2 signaling: Relevance for schizophrenia. PLoS ONE. 2017;12:e0175993.
Rosner M, Siegel AN, Valli AA, Fuchs AC, Hengstschläger AM. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids. 2010;38:223–8.
Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–92.
Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Am Physiol Soc. 2006. http://physiologyonline.physiology.org/content/nips/21/5/362.full.pdf. Accessed 21 Apr 2017.
Jhanwar-Uniyal M, Amin AG, Cooper JB, Das K, Schmidt MH, Murali R. Discrete signaling mechanisms of mTORC1 and mTORC2: connected yet apart in cellular and molecular aspects. Adv Biol Regul. 2017. https://doi.org/10.1016/j.jbior.2016.12.001.
Wiza C, Nascimento EBM, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab. 2012;302:E1453–60.
Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75.
Dos DS, Ali SM, Kim D-H, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302.
Fortin DA, Srivastava T, Soderling TR. Structural modulation of dendritic spines during synaptic plasticity. Neuroscience. 2012;18:326–41.
English J, Fan Y, Föcking M, Lopez L, Hryniewiecka M, Wynne K, et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry. 2015;5. https://doi.org/10.1038/tp.2015.119.
Laguesse S, Ron D. Protein translation and psychiatric disorders. Neurosci. 2019;25.
Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107.
Bhambhvani HP, Mueller TM, Simmons MS, Meador-Woodruff JH. Actin polymerization is reduced in the anterior cingulate cortex of elderly patients with schizophrenia. Transl Psychiatry. 2017;7:1278.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat Genet. 2004;36:131–7.
Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res. 2006;84:1–14.
Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. PNAS. 2011. https://doi.org/10.1073/pnas.1014715108.
Kim P, Scott MR, Meador-Woodruff JH. Abnormal ER quality control of neural GPI-anchored proteins via dysfunction in ER export processing in the frontal cortex of elderly subjects with schizophrenia. Transl Psychiatry. 2019;9:6.
Scott MR, Meador-Woodruff JH. Intracellular compartment-specific proteasome dysfunction in postmortem cortex in schizophrenia subjects. Mol Psychiatry. 2019;1–15. https://doi.org/10.1038/s41380-019-0359-7.
Kippe JM, Mueller TM, Haroutunian V, Meador-Woodruff JH. Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia. Schizophr Res. 2015;166:219–24.
Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005;75:303–8.
Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biol Psychiatry. 1986;21:650–6.
Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35:2110–9.
Hammond JC, Meador-Woodruff JH, Haroutunian V, McCullumsmith RE. Ampa receptor subunit expression in the endoplasmic reticulum in frontal cortex of elderly patients with schizophrenia. PLoS ONE. 2012;7:e39190.
Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013;24:688–91.
Pinner AL, Tucholski J, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased protein S-palmitoylation in dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2016;177:78–87.
Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–91.
Kim P, Scott MR, Meador-Woodruff JH. Abnormal expression of ER quality control and ER associated degradation proteins in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2018;197:484–91.
Xu Y, Yao Shugart Y, Wang G, Cheng Z, Jin C, Zhang K, et al. Altered expression of mRNA profiles in blood of early-onset schizophrenia. Sci Rep. 2016;6. https://doi.org/10.1038/srep16767.
Wen Z, Nam Nguyen H, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of mental disorders HHS Public Access. Nature. 2014;515:414–8.
Hoffman GE, Hartley BJ, Flaherty E, Ladran I, Gochman P, Ruderfer DM, et al. Transcriptional signatures of schizophrenia in hiPSC-derived NPCs and neurons are concordant with post-mortem adult brains. Nat Commun. 2017;8. https://doi.org/10.1038/s41467-017-02330-5.
Arion D, Huo Z, Enwright JF, Corradi JP, Tseng G, Lewis DA. Transcriptome alterations in prefrontal pyramidal cells distinguish schizophrenia from bipolar and major depressive disorders. Biol Psychiatry. 2017;82:594–600.
Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20:1397–405.
Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database. 2016;2016:baw100.
McGuire JL, Hammond JH, Yates SD, Chen D, Haroutunian V, Meador-Woodruff JH, et al. Altered serine/threonine kinase activity in schizophrenia. Brain Res. 2014;1568:42–54.
Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Publ Gr. 2013;16. https://doi.org/10.1038/nn.3546.
Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, et al. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700.
Bajestan SN, Sabouri AH, Nakamura M, Takashima H, Keikhaee MR, Behdani F, et al. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet Part B Neuropsychiatr Genet. 2006;141B:383–6.
Balu DT, Carlson GC, Talbot K, Kazi H, Hill-Smith TE, Easton RM, et al. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus. 2012;22:230–40.
Gong R, Park CS, Abbassi NR, Tang S-J. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–15.
Hsu W-L, Chung H-W, Wu C-Y, Wu H-I, Lee Y-T, Chen E-C, et al. Glutamate stimulates local protein synthesis in the axons of rat cortical neurons by activating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and metabotropic glutamate receptors. J Biol Chem. 2015;290:20748–60.
Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M, et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci. 2013;16:441–8.
Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, et al. Brain development and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61:379–84.
Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–47.
Thomas KT, Anderson BR, Shah N, Zimmer SE, Hawkins D, Valdez AN, et al. Inhibition of the schizophrenia-associated microRNA miR-137 disrupts Nrg1α neurodevelopmental signal transduction. Cell Rep. 2017;20:1–12.
Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009;20:1019–22.
Gururajan A, Van Den Buuse M. Is the mTOR-signalling cascade disrupted in Schizophrenia? J Neurochem. 2014;129:377–87.
Roh M-S, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM, et al. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med. 2007;39:353–60.
Pan B, Huang XF, Deng C. Aripiprazole and haloperidol activate GSK3β-dependent signalling pathway differentially in various brain regions of rats. Int J Mol Sci. 2016;17. https://doi.org/10.3390/ijms17040459.
Sarbassov DD, Bulgakova O, Bersimbaev RI, Shaiken T. Isolation of the mTOR complexes by affinity purification. In: Methods in molecular biology. Humana Press; 2012. p. 59–74.
Jain A, Arauz E, Aggarwal V, Ikon N, Chen J, Ha T. Stoichiometry and assembly of mTOR complexes revealed by single-molecule pulldown. Proc Natl Acad Sci USA. 2014;111:17833–8.
Dos DS, Ali SM, Kim D-H, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302.
De Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5:1512–26.
Macdonald ML, Garver M, Newman J, Sun Z, Kannarkat J, Salisbury R, et al. Synaptic proteome alterations in the primary auditory cortex of individuals with schizophrenia. JAMA Psychiatry. 2019. https://doi.org/10.1001/jamapsychiatry.2019.2974.
Acknowledgements
The authors would like to thank Dr Rosalinda Roberts and the Alabama Brain Collection for postmortem cortical samples used in assay development for western blot analyses and co-immunoprecipitation.
Author information
Authors and Affiliations
Contributions
RC and JMW and designed the study. RC executed experimental protocols, performed data calculations, statistical analyses, and literature searches. RC wrote the first draft of the manuscript, followed by editing by JMW. All authors contributed to and have approved the final manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chadha, R., Meador-Woodruff, J.H. Downregulated AKT-mTOR signaling pathway proteins in dorsolateral prefrontal cortex in Schizophrenia. Neuropsychopharmacol. 45, 1059–1067 (2020). https://doi.org/10.1038/s41386-020-0614-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0614-2
This article is cited by
-
Psychedelics and schizophrenia: a double-edged sword
Molecular Psychiatry (2025)
-
Neuronal alterations in AKT isotype expression in schizophrenia
Molecular Psychiatry (2025)
-
An increased copy number of glycine decarboxylase (GLDC) associated with psychosis reduces extracellular glycine and impairs NMDA receptor function
Molecular Psychiatry (2025)
-
“RNSP (Rannasangpei)” Rescued MK-801-induced Schizophrenia-like Behaviors in Mice via Oxidative Stress and BDNF-TrkB/Akt Pathway
Molecular Neurobiology (2024)
-
Assessment of Innovative Pharmacological Targets in Schizophrenia
Current Treatment Options in Psychiatry (2024)