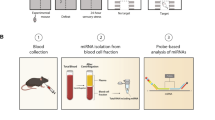
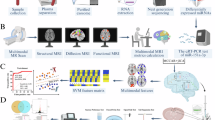
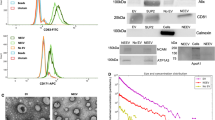
Abstract
Exosomal microRNAs (miRNAs) have been suggested to participate in the pathogenesis of neuropsychiatric diseases, but their role in major depressive disorder (MDD) is unknown. We performed a genome-wide miRNA expression profiling of blood-derived exosomes from MDD patients and control subjects and revealed the top differentially expressed exosomal miRNA, i.e. hsa-miR-139-5p (upregulation), had good performance to differentiate between MDD patients and controls. Tail vein injection of blood exosomes isolated from MDD patients into normal mice caused their depressive-like behaviors as determined by the forced swimming, tail suspension, and novelty suppressed feeding tests, and injection of blood exosomes isolated from healthy volunteers into unpredictable mild stress (CUMS)-treated mice alleviated their depressive-like behaviors. CUMS mice also showed significantly increased blood and brain levels of exosomal miR-139-5p. Furthermore, the depressive-like behaviors in CUMS-treated mice were rescued by intranasal injection of miR-139-5p antagomir, suggesting that increased exosomal miR-139-5p levels may mediate stress-induced depression-like behavior in mice. Both exosome treatment and miR-139-5p antagomir treatment increased hippocampal neurogenesis in the CUMS-treated mice, and treatment of exosome from MDD patients decreased hippocampal neurogenesis in the normal mice. The role of miR-139-5p in neurogenesis was validated by in vitro experiments, demonstrating that miR-139-5p is a negative regulator for neural stem cell proliferation and neuronal differentiation. Our findings together suggest that exosomes from patients with major depression caused depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Therefore, exosomal miRNAs are promising targets for the diagnosis and treatment of MDD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lopez JP, Kos A, Turecki G. Major depression and its treatment: microRNAs as peripheral biomarkers of diagnosis and treatment response. Curr Opin Psychiatry. 2018;31:7–16.
Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63.
Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:557–64.
MacQueen G, Santaguida P, Keshavarz H, Jaworska N, Levine M, Beyene J, et al. Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry. 2017;62:11–23.
Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci. 2019;73:613–27.
Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42.
Hunsberger JG, Austin DR, Chen G, Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromolecular Med. 2009;11:173–82.
Fries GR, Quevedo J. Exosomal microRNAs as potential biomarkers in neuropsychiatric disorders. Methods Mol Biol. 2018;1733:79–85.
Steinbichler TB, Dudas J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–81.
Tavakolizadeh J, Roshanaei K, Salmaninejad A, Yari R, Nahand JS, Sarkarizi HK, et al. MicroRNAs and exosomes in depression: potential diagnostic biomarkers. J Cell Biochem. 2018;119:3783–97.
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–66.
Du Y, Yu Y, Hu Y, Li XW, Wei ZX, Pan RY, et al. Genome-wide, integrative analysis implicates exosome-derived microRNA dysregulation in schizophrenia. Schizophr Bull. 2019;45:1257–66.
Vella LJ, Scicluna BJ, Cheng L, Bawden EG, Masters CL, Ang CS, et al. A rigorous method to enrich for exosomes from brain tissue. J Extracell Vesicles. 2017;6:1348885.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Wang L, Li XX, Chen X, Qin XY, Kardami E, Cheng Y, et al. Antidepressant-like effects of low- and high-molecular weight FGF-2 on chronic unpredictable mild stress mice. Front Mol Neurosci. 2018;11:377.
Cheng Y, Rodriguiz RM, Murthy SR, Senatorov V, Thouennon E, Cawley NX, et al. Neurotrophic factor-alpha1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Mol Psychiatry. 2015;20:744–54.
Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62.
MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–8.
Selvaraj P, Xiao L, Lee C, Murthy SR, Cawley NX, Lane M, et al. Neurotrophic factor-alpha1: a key Wnt-beta-Catenin dependent anti-proliferation factor and ERK-Sox9 activated inducer of embryonic neural stem cell differentiation to astrocytes in neurodevelopment. Stem Cells. 2017;35:557–71.
Coutinho de Almeida R, Ramos YFM, Mahfouz A, den Hollander W, Lakenberg N, Houtman E, et al. RNA sequencing data integration reveals an miRNA interactome of osteoarthritis cartilage. Ann Rheum Dis. 2019;78:270–7.
Wu C, Zhao Y, Liu Y, Yang X, Yan M, Min Y, et al. Identifying miRNA-mRNA regulation network of major depressive disorder in ovarian cancer patients. Oncol Lett. 2018;16:5375–82.
Jimenez-Fernandez S, Gurpegui M, Diaz-Atienza F, Perez-Costillas L, Gerstenberg M, Correll CU, et al. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76:1658–67.
Maffioletti E, Cattaneo A, Rosso G, Maina G, Maj C, Gennarelli M, et al. Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J Affect Disord. 2016;200:250–8.
Roy B, Dunbar M, Shelton RC, Dwivedi Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology. 2017;42:864–75.
Yuan H, Mischoulon D, Fava M, Otto MW. Circulating microRNAs as biomarkers for depression: many candidates, few finalists. J Affect Disord. 2018;233:68–78.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteom Bioinforma. 2015;13:17–24.
Tan L, Wu H, Liu Y, Zhao M, Li D, Lu Q. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity. 2016;49:357–65.
Liao FL, Tan L, Liu H, Wang JJ, Ma XT, Zhao B, et al. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharm Sin. 2018;39:552–60.
Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–72.
Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:168–84.
Fernandes MF, Mutch DM, Leri F. The relationship between fatty acids and different depression-related brain regions, and their potential role as biomarkers of response to antidepressants. Nutrients. 2017;9:298.
Ren Q, Wang ZZ, Chu SF, Xia CY, Chen NH. Gap junction channels as potential targets for the treatment of major depressive disorder. Psychopharmacology. 2018;235:1–12.
Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T, et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19:3422–31.
Qin XY, Cao C, Cawley NX, Liu TT, Yuan J, Loh YP, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: a meta-analysis study (N = 7277). Mol Psychiatry. 2017;22:312–20.
Egeland M, Guinaudie C, Du Preez A, Musaelyan K, Zunszain PA, Fernandes C, et al. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl Psychiatry. 2017;7:e1101.
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–61.
Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–78.
Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75.
Gadad BS, Jha MK, Czysz A, Furman JL, Mayes TL, Emslie MP, et al. Peripheral biomarkers of major depression and antidepressant treatment response: current knowledge and future outlooks. J Affect Disord. 2018;233:3–14.
Acknowledgements
We thank Dr. X. Zhang for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wei, ZX., Xie, GJ., Mao, X. et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacol. 45, 1050–1058 (2020). https://doi.org/10.1038/s41386-020-0622-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0622-2
This article is cited by
-
Potential MiRNA therapies for premature ovarian failure: new challenges and opportunities
Stem Cell Research & Therapy (2025)
-
Differentially expressed microRNAs in human depression: a systematic review of case-control and longitudinal studies
BMC Psychiatry (2025)
-
Exosomes from high-altitude cerebral edema patients induce cognitive dysfunction by altering oxidative stress responses in mice
Translational Psychiatry (2025)
-
Alterations in miR-151a-3p of plasma-derived exosomes and associated multimodal neuroimaging patterns in major depressive disorder
Molecular Psychiatry (2025)
-
Hypoxic preconditioning modulates BDNF signaling to alleviate depression-like behaviors in mice and its whole transcriptome sequencing analysis
Scientific Reports (2025)