Abstract
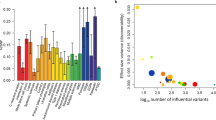
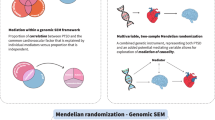
Inflammatory markers like C-reactive protein (CRP) have been associated with post-traumatic stress disorder (PTSD) and traumatic experiences, but the underlying mechanisms are unclear. We investigated the relationship among serum CRP, PTSD, and traits related to traumatic events and social support using genetic association data from the Psychiatric Genomics Consortium (23,185 PTSD cases and 151,309 controls), the UK Biobank (UKB; up to 117,900 individuals), and the CHARGE study (Cohorts for Heart and Aging Research in Genomic Epidemiology, 148,164 individual). Linkage disequilibrium score regression, polygenic risk scoring, and two-sample Mendelian randomization (MR) analyses were used to investigate genetic overlap and causal relationships. Genetic correlations of CRP were observed with PTSD (rg = 0.16, p = 0.026) and traits related to traumatic events, and the presence of social support (−0.28 < rg < 0.20; p < 0.008). We observed a bidirectional association between CRP and PTSD (CRP → PTSD: β = 0.065, p = 0.015; PTSD → CRP: β = 0.008, p = 0.009). CRP also showed a negative association with the “felt loved as a child” trait (UKB, β = −0.017, p = 0.008). Owing to the known association of socioeconomic status (SES) on PTSD, a multivariable MR was performed to investigate SES as potential mediator. We found that household income (univariate MR: β = −0.22, p = 1.57 × 10−7; multivariate MR: β = −0.17, p = 0.005) and deprivation index (univariate MR: β = 0.38, p = 1.63 × 10−9; multivariate MR: β = 0.27, p = 0.016) were driving the causal estimates of “felt loved as a child” and CRP on PTSD. The present findings highlight a bidirectional genetic association between PTSD and CRP, also suggesting a potential role of SES in the interplay between childhood support and inflammatory processes with respect to PTSD risk.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
North CS, Suris AM, Smith RP, King RV. The evolution of PTSD criteria across editions of DSM. Ann Clin Psychiatry. 2016;28:197–208.
Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353–62.
Vashist SK, Venkatesh AG, Marion Schneider E, Beaudoin C, Luppa PB, Luong JH. Bioanalytical advances in assays for C-reactive protein. Biotechnol Adv. 2016;34:272–90.
Rosen RL, Levy-Carrick N, Reibman J, Xu N, Shao Y, Liu M, et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J Psychiatr Res. 2017;89:14–21.
Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, et al. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–8.
Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, et al. Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry. 2014;4:e413.
Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–12.
Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–31.
Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42:117–31.
Glaus J, von Kanel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: results from a large longitudinal population-based study. Depress Anxiety. 2018;35:360–71.
Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, et al. Association of prospective risk for chronic PTSD symptoms with low TNFalpha and IFNgamma concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry. 2019. https://doi.org/10.1176/appi.ajp.2019.19010039.
Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav Immun. 2018;69:203–09.
Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol Psychiatry. 2017;82:875–84.
Bielas H, Meister-Langraf RE, Schmid JP, Barth J, Znoj H, Schnyder U, et al. C-reactive protein as a predictor of posttraumatic stress induced by acute myocardial infarction. Gen Hosp Psychiatry. 2018;53:125–30.
Farr OM, Ko BJ, Joung KE, Zaichenko L, Usher N, Tsoukas M, et al. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovasc Dis. 2015;25:479–88.
O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–74.
Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol Psychiatry. 2019. https://doi.org/10.1016/j.biopsych.2019.11.005.
Evans DM, Davey Smith G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet. 2015;16:327–50.
Ravera S, Carrasco N, Gelernter J, Polimanti R. Phenomic impact of genetically-determined euthyroid function and molecular differences between thyroid disorders. J Clin Med. 2018;7:E296.
Polimanti R, Peterson RE, Ong JS, MacGregor S, Edwards AC, Clarke TK, et al. Evidence of causal effect of major depression on alcohol dependence: findings from the psychiatric genomics consortium. Psychol Med. 2019;49:1218–26.
Nievergelt CM, Maihofer AX, Klengel T, Atkinson E, Chen CY, Choi K, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9.
Ligthart S, Vaez A, Vosa U, Stathopoulou MG, de Vries PS, Prins BP, et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet. 2018;103:691–706.
Prag P, Mills MC, Wittek R. Subjective socioeconomic status and health in cross-national comparison. Soc Sci Med. 2016;149:84–92.
Stepanikova I, Bateman LB, Oates GR. Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. Am J Prev Med. 2017;52(1S1):S63–76.
Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35:407–11.
Polimanti R, Ratanatharathorn A, Maihofer AX, Choi KW, Stein MB, Morey RA, et al. Association of economic status and educational attainment with posttraumatic stress disorder: a Mendelian randomization study. JAMA Netw Open. 2019;2:e193447.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Logue MW, Amstadter AB, Baker DG, Duncan L, Koenen KC, Liberzon I, et al. The psychiatric genomics consortium posttraumatic stress disorder workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology. 2015;40:2287–97.
Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, et al. Mental health in UK Biobank Revised. medRxiv. 2019:19001214.
Hill WD, Davies NM, Ritchie SJ, Skene NG, Bryois J, Bell S, et al. Genome-wide analysis identifies molecular systems and 149 genetic loci associated with income. Nat Commun. 2019;10:5741.
Abdellaoui A, Hugh-Jones D, Yengo L, Kemper KE, Nivard MG, Veul L, et al. Genetic correlates of social stratification in Great Britain. Nat Hum Behav. 2019;3:1332–42.
Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31:1466–8.
Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607.
Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. 2017;4:330–45.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36:1783–802.
Davey Smith G, Davies NM, Dimou N, Egger M, Gallo V, Golub R, et al. STROBE-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ Prepr. 2019;7:e27857v1.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. arXiv. 2019:1801.09652.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–98.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28:30–42.
Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–60.
Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42:254–70.
Wang Z, Young MR. PTSD, a disorder with an immunological component. Front Immunol. 2016;7:219.
Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci USA. 2016;113:578–83.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Fernandes BS, Steiner J, Molendijk ML, Dodd S, Nardin P, Goncalves CA, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:1147–56.
Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem. 2012;30:1109–19.
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306.
Felger JC, Haroon E, Miller AH. What’s CRP got to do with it? Tackling the complexities of the relationship between CRP and depression. Brain Behav Immun. 2018;73:163–64.
Shen J, Ordovas JM. Impact of genetic and environmental factors on hsCRP concentrations and response to therapeutic agents. Clin Chem. 2009;55:256–64.
O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–9.
Stringhini S, Polidoro S, Sacerdote C, Kelly RS, van Veldhoven K, Agnoli C, et al. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int J Epidemiol. 2015;44:1320–30.
Lin YH, Jen MH, Chien KL. Association between life-course socioeconomic position and inflammatory biomarkers in older age: a nationally representative cohort study in Taiwan. BMC Geriatr. 2017;17:201.
Lakey B, Orehek E. Relational regulation theory: a new approach to explain the link between perceived social support and mental health. Psychol Rev. 2011;118:482–95.
Hakulinen C, Pulkki-Raback L, Jokela M, E Ferrie J, Aalto AM, Virtanen M. et al. Structural and functional aspects of social support as predictors of mental and physical health trajectories: Whitehall II cohort study. J Epidemiol Community Health. 2016;70:710–5.
Lee JS. Perceived social support functions as a resilience in buffering the impact of trauma exposure on PTSD symptoms via intrusive rumination and entrapment in firefighters. PLoS ONE. 2019;14:e0220454.
De Nutte L, Okello J, Derluyn I. Social relationships and social support among post-war youth in Northern Uganda. Int J Psychol. 2017;52:291–9.
Shimanoe C, Hara M, Nishida Y, Nanri H, Otsuka Y, Horita M, et al. Coping strategy and social support modify the association between perceived stress and C-reactive protein: a longitudinal study of healthy men and women. Stress. 2018;21:237–46.
Elliot AJ, Heffner KL, Mooney CJ, Moynihan JA, Chapman BP. Social relationships and inflammatory markers in the MIDUS cohort: the role of age and gender differences. J Aging Health. 2018;30:904–23.
Martschenko D, Trejo S, Domingue BW. Genetics and education: recent developments in the context of an ugly history and an uncertain future. AERA Open. 2019;5:2332858418810516.
Trejo S, Domingue BW. Genetic nature or genetic nurture? Introducing social genetic parameters to quantify bias in polygenic score analyses. Biodemography Soc Biol. 2018;64:187–215.
Author information
Authors and Affiliations
Contributions
Conception and design: CMC, FRW, RP; acquisition of data: CMC, RP; analysis and interpretation of data: CMC, FRW, AXM, DJS, MBS, JAS, SMJH, CMN, KCK, JG, SIB, and RP; drafting the article: CMC, RP; revising the article critically for important intellectual content: CMC, FRW, AXM, DJS, MBS, JAS, SMJH, CMN, KCK, JG, SIB, and RP; final approval of the version to be published: CMC, FRW, AXM, DJS, MBS, JAS, SMJH, CMN, KCK, JG, SIB, and RP.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Muniz Carvalho, C., Wendt, F.R., Maihofer, A.X. et al. Dissecting the genetic association of C-reactive protein with PTSD, traumatic events, and social support. Neuropsychopharmacol. 46, 1071–1077 (2021). https://doi.org/10.1038/s41386-020-0655-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0655-6
This article is cited by
-
Causal associations between posttraumatic stress disorder and type 2 diabetes
Diabetology & Metabolic Syndrome (2025)
-
Effects of genetically predicted posttraumatic stress disorder on autoimmune phenotypes
Translational Psychiatry (2024)
-
Epigenome-wide association studies identify novel DNA methylation sites associated with PTSD: a meta-analysis of 23 military and civilian cohorts
Genome Medicine (2024)
-
Psychological and biological mechanisms linking trauma with cardiovascular disease risk
Translational Psychiatry (2023)
-
An analysis on history of childhood adversity, anxiety, and chronic pain in adulthood and the influence of inflammatory biomarker C-reactive protein
Scientific Reports (2023)