Abstract
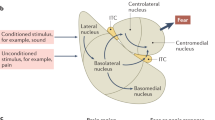
Posttraumatic stress disorder (PTSD) may develop when mechanisms for making accurate distinctions about threat relevance have gone awry. Generalization across conceptually related objects has been hypothesized based on clinical observation in PTSD, but the neural mechanisms remain unexplored. Recent trauma-exposed military veterans (n = 46) were grouped into PTSD (n = 23) and non-PTSD (n = 23). Participants learned to generalize fear across conceptual categories (animals or tools) of semantically related items that were partially reinforced by shock during functional magnetic resonance imaging. Conditioned fear learning was quantified by shock expectancy and skin conductance response (SCR). Relative to veteran controls, PTSD subjects exhibited a stronger neural response associated with fear generalization to the reinforced object category in the striatum, anterior cingulate cortex, amygdala, occipitotemporal cortex, and insula (Z > 2.3; p < 0.05; whole-brain corrected). Based on SCR, both groups generalized the shock contingency to the reinforced conceptual category, but learning was not significantly different between groups. We found that PTSD was associated with an enhanced neural response in fronto-limbic, midline, and occipitotemporal regions to a learned representation of threat that is based on previously established conceptual knowledge of the relationship between basic-level exemplars within a semantic category. Behaviorally, veterans with PTSD were somewhat slower to differentiate threat and safety categories as compared with trauma-exposed veteran controls owing in part to an initial overgeneralized behavioral response to the safe category. These results have implications for understanding how fear spreads across semantically related concepts in PTSD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Dunsmoor JE, Paz R. Fear generalization and anxiety: Behavioral and neural mechanisms. Biol Psychiatry. 2015;78:336–43.
LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–76.
Morey R, Dunsmoor J, Haswell C, Brown V, Vora A, Weiner J, et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl psychiatry. 2015;5:e700.
Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, et al. Neural substrates of overgeneralized conditioned fear in ptsd. Am J psychiatry:appi ajp. 2016;174:2016.15121549.
Ehlers A. Understanding and treating unwanted trauma memories in posttraumatic stress disorder. J Psychol. 2015;218:141–5.
Vervoort E, Vervliet B, Bennett M, Baeyens F. Generalization of human fear acquisition and extinction within a novel arbitrary stimulus category. PloS ONE. 2014;9:e96569.
Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav Ther. 2015;46:561–82.
Dunsmoor JE, Martin A, LaBar KS. Role of conceptual knowledge in learning and retention of conditioned fear. Biol Psychol. 2012;89:300–5.
Dunsmoor JE, Kragel PA, Martin A, Labar KS. Aversive learning modulates cortical representations of object categories. Cereb Cortex. 2014;24:2859–72.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769.
Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, et al. The association of ptsd symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress. 2017;1:2470547017724069.
Sheynin J, Liberzon I. Circuit dysregulation and circuit-based treatments in posttraumatic stress disorder. Neurosci Lett. 2017;649:133–8.
Fitzgerald JM, DiGangi JA, Phan KL. Functional neuroanatomy of emotion and its regulation in ptsd. Harv Rev psychiatry. 2018;26:116–28.
Clausen AN, Clarke E, Phillips RD, Haswell C, Morey RA. Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military veterans. Neuropsychopharmacology. 2019;45:1–9.
Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47.
Lanius R, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in ptsd: Heterogeneity of response to symptom provocation. J Psychiatr Res. 2006;40:709–29.
Neumeister P, Feldker K, Heitmann CY, Helmich R, Gathmann B, Becker MP, et al. Interpersonal violence in posttraumatic women: Brain networks triggered by trauma-related pictures. Soc Cogn Affect Neurosci. 2017;12:555–68.
Brancu M, Wagner HR, Morey RA, Beckham JC, Calhoun PS, Tupler LA, et al. The post‐deployment mental health (pdmh) study and repository: a multi-site study of us afghanistan and iraq era veterans. Int. J. Methods Psychiatr. Res. 26 (2017).
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered ptsd scale. J Trauma Stress. 1995;8:75–90.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (audit): who collaborative project on early detection of persons with harmful alcohol consumption–ii. Addiction. 1993;88:791–804.
Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J psychiatry. 1994;151:1132.
Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the traumatic life events questionnaire. Psychol. Assess. 2000;12:210–24.
Beck AT, Steer RA, Brown GK (eds). Manual for the beck depression inventory-ii. San Antonio, TX: Psychological Corp; 1996.
Skinner HA. The drug abuse screening test. Addictive Behav. 1982;7:363–71.
Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522.
Dunsmoor JE, Mitroff SR, Labar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Mem. 2009;16:460–9.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural mr image analysis and implementation as fsl. Neuroimage. 2004;23:S208–19.
Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73.
Eklund A, Nichols TE, Knutsson H. Cluster failure: why fmri inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016:113, 7900–5.
Han H, Glenn AL, Dawson KJ. Evaluating alternative correction methods for multiple comparison in functional neuroimaging research. Brain Sci. 2019;9:198.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gppi): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis–connecting the branches of systems neuroscience. Front Syst Neurosci. 2008;2:4.
Deen B, Richardson H, Dilks DD, Takahashi A, Keil B, Wald LL, et al. Organization of high-level visual cortex in human infants. Nat Commun. 2017;8:13995.
Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8.
Diedenhofen B, Musch J. Cocor: a comprehensive solution for the statistical comparison of correlations. PloS ONE. 2015;10:e0121945.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83.
Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak R, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626.
Huth AG, Nishimoto S, Vu AT, Gallant JL. A continuous semantic space describes the representation of thousands of object and action categories across the human brain. Neuron. 2012;76:1210–24.
Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96.
Gilboa A, Marlatte H. Neurobiology of schemas and schema-mediated memory. Trends Cogn Sci. 2017;21:618–31.
Ehlers A, Hackmann A, Steil R, Clohessy S, Wenninger K, Winter H. The nature of intrusive memories after trauma: The warning signal hypothesis. Behav Res Ther. 2002;40:995–1002.
Striedter GF. Evolution of the hippocampus in reptiles and birds. J Comp Neurol. 2016;524:496–517.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82.
Dunsmoor JE, White AJ, LaBar KS. Conceptual similarity promotes generalization of higher order fear learning. Learn Mem. 2011;18:156–60.
Onat S, Büchel C. The neuronal basis of fear generalization in humans. Nat Neurosci. 2015;18:1811–8.
Preusser F, Margraf J, Zlomuzica A. Generalization of extinguished fear to untreated fear stimuli after exposure. Neuropsychopharmacology. 2017;42:2545.
Dunsmoor JE, Murphy GL. Categories, concepts, and conditioning: how humans generalize fear. Trends Cogn Sci. 2015;19:73–7.
Acknowledgements
The VA Mid-Atlantic MIRECC Workgroup contributors for this paper include: Mira Brancu, PhD, Jean C. Beckham, PhD, Patrick S. Calhoun, PhD, Eric Dedert, PhD, Eric B. Elbogen, PhD, John A. Fairbank, PhD, Robin A. Hurley, MD, Jason D. Kilts, PhD, Nathan A. Kimbrel, PhD, Angela Kirby, MS, Christine E. Marx, MD, MA, Scott D. McDonald, PhD, Scott D. Moore, MD, PhD, Jennifer C. Naylor, PhD, Jared Rowland, PhD, Cindy Swinkels, PhD, Steven T. Szabo, MD, PhD, Katherine H. Taber, PhD., Larry A. Tupler, PhD, Elizabeth E. Van Voorhees, PhD, H. Ryan Wagner, Ph.D., Ruth E. Yoash-Gantz, PsyD. This research was supported by the US Department of Veterans Affairs (VA) Mid-Atlantic Mental Illness Research, Education, and Clinical Center (MIRECC) core funds of the Department of Veterans Affairs Office of Mental Health Services. Dr. Morey also received financial support from the VA Office of Research and Development (5I01CX000748-01, 5I01CX000120-02). Additional financial support was provided by the National Institute for Neurological Disorders and Stroke (R01NS086885-01A1). Writing of this manuscript was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Durham VA Health Care System, and the Department of Veterans Affairs Mid-Atlantic MIRECC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Consortia
Contributions
R.A.M. wrote the manuscript, contributed to data analysis, interpretation of the data, and acquisition of data, gave final approval for the version to be published and is accountable for all aspects of the work including accuracy and integrity. C.C.H. made substantial contributions to data analysis, interpretation of the data, acquisition of data, and gave final approval for the version to be published. D.S. contributed to data analysis, interpretation of the data, and acquisition of data, gave final approval for the version to be published. M.A.M. Workgroup contributed to the acquisition of data, gave final approval for the version to be published. J.E.D. made substantial contributions to the conception and design, contributed to the interpretation of the data, gave final approval for the version to be published. K.S.L. made substantial contributions to the conception and design, interpretation of the data, and writing the manuscript, gave final approval for the version to be published, and is accountable for all aspects of the work including accuracy and integrity.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of members and their affiliations are listed at the end of the paper.
Supplementary information
Rights and permissions
About this article
Cite this article
Morey, R.A., Haswell, C.C., Stjepanović, D. et al. Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacol. 45, 1380–1389 (2020). https://doi.org/10.1038/s41386-020-0661-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0661-8
This article is cited by
-
Traumatic stress alters neural reactivity to visual stimulation
NPP—Digital Psychiatry and Neuroscience (2025)
-
A visual generalization gradient of conceptual stimuli based on fear acquisition in visual and auditory modalities
npj Science of Learning (2025)
-
Impaired mnemonic pattern separation associated with PTSD symptoms paradoxically improves with regular cannabis use
npj Mental Health Research (2025)
-
How Psychedelics Modulate Multiple Memory Mechanisms in Posttraumatic Stress Disorder
Drugs (2024)
-
The influence of being left behind on fear acquisition and academic performance—a study of left-behind children
Current Psychology (2023)