Abstract
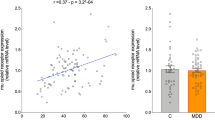
Major depressive disorder is associated with lowered mood, anxiety, anhedonia, sleep problems, and cognitive impairments. Many of these functions are regulated by μ-opioid receptor (MOR) system. Preclinical, in vivo, and post-mortem studies have however yielded inconclusive results regarding the role of the MOR in depression and anxiety. Moreover, it is not known whether alterations in MOR are already present in subclinical depression and anxiety. In a large-scale retrospective cross-sectional study we pooled data from 135 (113 males and 22 females) healthy subjects whose brain’s MOR availability was measured with positron emission tomography (PET) using an agonist radioligand [11C]carfentanil that has high affinity for MORs. Depressive and anxious symptomology was addressed with BDI-II and STAI-X questionnaires, respectively. Both anxiety and depression scores in the subclinical range were negatively associated with MOR availability in cortical and subcortical areas, notably in amygdala, hippocampus, ventral striatum, and orbitofrontal and cingulate cortices. We conclude that dysregulated MOR availability is involved in altered mood and pathophysiology of depression and anxiety disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nummenmaa L, Tuominen LJ. Opioid system and human emotions. Br J Pharmacol. 2018;175:2737–49.
van Steenbergen H, Eikemo M, Leknes S. The role of the opioid system in decision making and cognitive control: a review. Cogn Affect Behav Neurosci. 2019;19:435–58.
Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206.
Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, et al. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64:651–60.
Davis M. Morphine and naloxone: effects on conditioned fear as measured with the potentiated startle paradigm. Eur J Pharmacol. 1979;54:341–7.
Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109:631–41.
Zarrindast M-R, Babapoor-Farrokhran S, Babapoor-Farrokhran S, Rezayof A. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 2008;82:1175–81.
Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131:1171–96.
Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, et al. Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology. 2016;41:2344–51.
Robinson SA, Erickson RL, Browne CA, Lucki I. A role for the mu opioid receptor in the antidepressant effects of buprenorphine. Behav Brain Res. 2016;319:96–103.
Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57.
Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28:593–5.
Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108–12.
Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled. Trial Am J Psychiat. 2015;173:491–8.
Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–7.
Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009;65:438–40.
Saxe G, Stoddard F, Courtney D, Cunningham K, Chawla N, Sheridan R, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry. 2001;40:915–21.
Zubieta JK, Ketter TA, Bueller JA, Xu YJ, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–53.
Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, et al. Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry. 2013;18:1211–7.
Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–208.
Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry. 2015;20:193–200.
Tuominen L, Salo J, Hirvonen J, Nagren K, Laine P, Melartin T, et al. Temperament trait Harm Avoidance associates with mu-opioid receptor availability in frontal cortex: A PET study using C-11 carfentanil. Neuroimage. 2012;61:670–6.
Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, et al. Altered central micro-opioid receptor binding after psychological trauma. Biol Psychiatry. 2007;61:1030–8.
Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–6.
Gabilondo AM, Meana JJ, García-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–50.
Scarr E, Money TT, Pavey G, Neo J, Dean B. Mu opioid receptor availability in people with psychiatric disorders who died by suicide: a case control study. BMC Psychiatry. 2012;12:126.
Zalsman G, Molcho A, Huang Y, Dwork A, Li S, Mann JJ. Postmortem mu-opioid receptor binding in suicide victims and controls. J Neural Transm. 2005;112:949–54.
Karsten J, Hartman CA, Smit JH, Zitman FG, Beekman ATF, Cuijpers P, et al. Psychiatric history and subthreshold symptoms as predictors of the occurrence of depressive or anxiety disorder within 2 years. Br J Psychiatry. 2011;198:206–12.
Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100.
Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
Frost JJ, Wagner HN Jr., Dannals RF, Ravert HT, Links JM, Wilson AA, et al. Imaging opiate receptors in the human brain by positron tomography. J Comput Assist Tomogr. 1985;9:231–6.
Karjalainen T, Tuominen L, Manninen S, Kalliokoski K, Nuutila P, Jääskeläinen IP, et al. Behavioural activation system sensitivity is associated with cerebral μ-opioid receptor availability. Soc Cogn Affect Neurosci. 2016;11:1310–6.
Nummenmaa L, Manninen S, Tuominen L, Hirvonen J, Kalliokoski KK, Nuutila P, et al. Adult attachment style Is associated with cerebral μ-opioid receptor availability in humans. Hum Brain Mapp. 2015;36:3621–8.
Karjalainen T, Santavirta S, Kantonen T, Tuisku J, Tuominen L, Hirvonen J, et al. Magia: robust automated modeling and image processing toolbox for PET neuroinformatics. Front Neuroinform. 2020:604835.
Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J, et al. Interindividual variability and lateralization of µ-opioid receptors in the human brain. Neuroimage. 2020;217:116922.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Giedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6:279–87.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–8.
Frost JJ, Douglass KH, Mayberg HS, Dannals RF, Links JM, Wilson AA. Multicompartmental analysis of [11C]-carfentanil binding to opiate receptors in humans measured by positron emission tomography. J Cereb Blood Flow Metab. 1989;9:398–409.
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15.
Hayakawa YK, Sasaki H, Takao H, Mori H, Hayashi N, Kunimatsu A, et al. Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. J Affect Disord. 2013;144:263–8.
Kraus C, Hahn A, Savli M, Kranz GS, Baldinger P, Hoeflich A, et al. Serotonin-1A receptor binding is positively associated with gray matter volume—a multimodal neuroimaging study combining PET and structural MRI. Neuroimage. 2012;63:1091–8.
Woodward ND, Zald DH, Ding Z, Riccardi P, Ansari MS, Baldwin RM, et al. Cerebral morphology and dopamine D2/D3 receptor distribution in humans: a combined [18F]fallypride and voxel-based morphometry study. Neuroimage. 2009;46:31–8.
Manninen S, Karjalainen T, Tuominen LJ, Hietala J, Kaasinen V, Joutsa J, et al. Cerebral grey matter density is associated with neuroreceptor and neurotransporter availability: a combined PET and MRI study. 2020. https://www.biorxiv.org/content/10.1101/2020.01.29.924530v1.full.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35.
Bürkner P-C. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80:1–28.
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. Chapman and Hall/CRC; Boca Raton, FL, 2013.
Gabry J, Simpson D, Vehtari A, Betancourt M, Gelman A. Visualization in Bayesian workflow. J R Stat Soc Ser A. 2019;182:389–402.
Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press; New York, NY, 2006.
Tuominen L, Nummenmaa L, Keltikangas-Järvinen L, Raitakari O, Hietala J. Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Hum Brain Mapp. 2014;35:1875–84.
Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14:321.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655.
Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response. Data Am J Psychiatry. 2012;169:693–703.
Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–8.
Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology. 2008;200:105–15.
Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–9.
Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–7.
Fox ME, Lobo MK. The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry 2019;24:1798–815.
Manninen S, Tuominen L, Dunbar RIM, Karjalainen T, Hirvonen J, Arponen E, et al. Social laughter triggers endogenous opioid release in humans. J Neurosci. 2017;37:6125–31.
Tuulari JJ, Tuominen L, de Boer FE, Hirvonen J, Helin S, Nuutila P, et al. Feeding releases endogenous opioids in humans. J Neurosci. 2017;37:8284–91.
Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta JK. Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. J Clin Endocrinol Metab. 2015;100:3193–201.
Saanijoki T, Tuominen L, Tuulari JJ, Nummenmaa L, Arponen E, Kalliokoski K, et al. Opioid release after high-intensity interval training in healthy human subjects. Neuropsychopharmacology. 2017;43:246–54.
Nummenmaa L, Tuominen L, Dunbar R, Hirvonen J, Manninen S, Arponen E, et al. Social touch modulates endogenous µ-opioid system activity in humans. Neuroimage. 2016;138:242–7.
Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–20.
Karjalainen T, Seppala K, Glerean E, Karlsson HK, Lahnakoski JM, Nuutila P, et al. Opioidergic regulation of emotional arousal: a combined PET-fMRI study. Cereb Cortex. 2019;29:4006–16.
Karjalainen T, Karlsson HK, Lahnakoski JM, Glerean E, Nuutila P, Jaaskelainen IP, et al. Dissociable roles of cerebral mu-opioid and type 2 dopamine receptors in vicarious pain: a combined PET-fMRI study. Cereb Cortex. 2017;27:1–10.
Heiskanen T, Leppä M, Suvilehto J, Akural E, Larinkoski T, Jääskeläinen IP, et al. The opioid agonist remifentanil increases subjective pleasure during emotional stimulation. Br J Anaesthesiol. 2019;122:E216–E219.
Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407.
Peciña M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, Zubieta J-K. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry 2019;24:576–87.
Weerts EM, McCaul ME, Kuwabara H, Yang XJ, Xu XQ, Dannals RF, et al. Influence of OPRM1 Asn(40)Asp variant (A118G) on C-11 carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol. 2013;16:47–53.
Author information
Authors and Affiliations
Contributions
Acquired the data: TK, VK, JJ, PN, KK, JH, and JR. Analyzed the data: LN, ToK, JI, TK, and JT. Designed the study: LN, ToK, and JR. Wrote the paper: all authors.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nummenmaa, L., Karjalainen, T., Isojärvi, J. et al. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacol. 45, 1953–1959 (2020). https://doi.org/10.1038/s41386-020-0725-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0725-9
This article is cited by
-
Role of astrocytic mu-opioid receptors of the ventrolateral periaqueductal gray in modulating anxiety-like responses
Behavioral and Brain Functions (2025)
-
Anorexia nervosa is associated with higher brain mu-opioid receptor availability
Molecular Psychiatry (2025)
-
Childhood family environment and μ-opioid receptor availability in vivo in adulthood
Neuropsychopharmacology (2025)
-
Endogenous opioid receptor system mediates costly altruism in the human brain
Communications Biology (2024)