Abstract
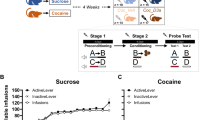
Cocaine is known to increase brain dopamine at supranormal levels in comparison to alternative nondrug rewards. According to the dopamine hypothesis of addiction, this abnormally large dopamine response would explain why cocaine use is initially highly rewarding and addictive. Though resting on solid neuroscientific foundations, this hypothesis has nevertheless proven difficult to reconcile with research on cocaine choice in experimental animals. When facing a choice between an intravenous bolus of cocaine and a nondrug alternative (e.g., sweet water), both delivered immediately after choice, rats do not choose the drug, as would be predicted, but instead develop a strong preference for the nondrug alternative. Here we report evidence that reconciles this finding with the dopamine hypothesis of addiction. First, a systematic literature analysis revealed that the delays of effects of intravenous cocaine on nucleus accumbens dopamine are of the order of tens of seconds and are considerably longer than those of nondrug reward. Second, this was confirmed by measuring response times to cocaine omission during self-administration as a behavioral proxy of these delays. Finally, when the influence of the drug delays was reduced during choice by adding an increasing delay to both the drug and nondrug rewards, rats shifted their choice to cocaine. Overall, this study suggests that cocaine is indeed supranormal in reward magnitude, as postulated by the dopamine hypothesis of addiction, but is less preferred during choice because its pharmacokinetics makes it an inherently more delayed reward than the alternative. Reframing previous drug choice studies in rats as intertemporal choice studies reveals that the discounting effects of delays spare no rewards, including supranormal ones, and that during choice, pharmacokinetics trumps pharmacodynamics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharm. 1999;375:13–30.
Keiflin R, Janak PH. Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron. 2015;88:247–63.
Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94.
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62.
Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46.
Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–7.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13.
Ahmed SH. Trying to make sense of rodents’ drug choice behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt A):3–10.
Ahmed SH. Individual decision-making in the causal pathway to addiction: contributions and limitations of rodent models. Pharm Biochem Behav. 2018;164:22–31.
Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5:e11592.
Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698.
Madsen HB, Ahmed SH. Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol. 2015;20:433–44.
Bagley JR, Adams J, Bozadjian RV, Bubalo L, Ploense KL, Kippin TE. Estradiol increases choice of cocaine over food in male rats. Physiol Behav. 2019;203:18–24.
Kearns DN, Kim JS, Tunstall BJ, Silberberg A. Essential values of cocaine and non-drug alternatives predict the choice between them. Addict Biol. 2017;22:1501–14.
Tunstall BJ, Kearns DN. Reinstatement in a cocaine versus food choice situation: reversal of preference between drug and non-drug rewards. Addict Biol. 2014;19:838–48.
Tunstall BJ, Kearns DN. Sign-tracking predicts increased choice of cocaine over food in rats. Behav Brain Res. 2015;281:222–8.
Tunstall BJ, Kearns DN. Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol. 2016;21:282–93.
Tunstall BJ, Riley AL, Kearns DN. Drug specificity in drug versus food choice in male rats. Exp Clin Psychopharmacol. 2014;22:364–72.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73.
Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol. 2015;20:913–26.
Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. The anterior insular cortex–>central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron. 2017;96:414–27. e8
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Schwartz LP, Kim JS, Silberberg A, Kearns DN. Heroin and saccharin demand and preference in rats. Drug Alcohol Depend. 2017;178:87–93.
Vandaele Y, Vouillac-Mendoza C, Ahmed SH. Inflexible habitual decision-making during choice between cocaine and a nondrug alternative. Transl Psychiatry. 2019;9:109.
Venniro M, Russell TI, Zhang M, Shaham Y. Operant social reward decreases incubation of heroin craving in male and female rats. Biol Psychiatry. 2019;86:848–56.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–29.
Welling PG. Pharmacokinetics: processes and mathematics. Washington, DC: American Chemical Society; 1986.
Pan HT, Menacherry S, Justice JB Jr. Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochemistry. 1991;56:1299–306.
Lau CE, Sun L. The pharmacokinetic determinants of the frequency and pattern of intravenous cocaine self-administration in rats by pharmacokinetic modeling. Drug Metab Disposition. 2002;30:254–61.
Wise RA, Kiyatkin EA. Differentiating the rapid actions of cocaine. Nat Rev Neurosci. 2011;12:479–84.
Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96.
Mazur JE. Choice, delay, probability, and conditioned reinforcement. Anim Learn Behav. 1997;25:131–47.
Stevens JE, Stephens DW. The adaptive nature of impulsivity. In: Madden GJ, Bickel WK, editors. Impulsivity: the behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. p. 361–88.
Vanderveldt A, Oliveira L, Green L. Delay discounting: pigeon, rat, human – does it matter? J Exp Psychol: Anim Learn cognition. 2016;42:141–62.
van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren LJMJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73.
Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacol (Berl). 1996;128:161–70.
Richards JB, Mitchell SH, De Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–66.
Logan FA. Decision making by rats: delay versus amount of reward. J Comp Physiol Psychol. 1965;59:1–12.
Bradshaw CM, Szabadi E. Choice between delayed reinforcers in a discrete-trials schedule: the effect of deprivation level. Quaterly J Exp Psychol. 1992;44B:1–16.
Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacol (Berl). 2000;152:362–75.
Peterson JR, Hill CC, Kirkpatrick K. Measurement of imulsive choice in rats: same- and alternate-form test-retest reliability and temporal tracking. J Exp Anal Behav. 2015;103:166–79.
Panlilio LV, Secci ME, Schindler CW, Bradberry CW. Choice between delayed food and immediate opioids in rats: treatment effects and individual differences. Psychopharmacol (Berl). 2017;234:3361–73.
Secci ME, Factor JA, Schindler CW, Panlilio LV. Choice between delayed food and immediate oxycodone in rats. Psychopharmacol (Berl). 2016;233:3977–89.
Wise RA, Kiyatkin EA. On the speed of cocaine. Nat Rev Neurosci. 2011;12:700.
Aragona BJ. The regional specificity of rapid actions of cocaine. Nat Rev Neurosci. 2011;12:700.
Green L, Estle SJ. Preference reversals with food and water reinforcers in rats. J Exp Anal Behav. 2003;79:233–42.
Ainslie GW, Herrnstein RJ. Preference reversal and delayed reinforcement. Anim Learn Behav. 1981;9:476–82.
Green L, Fisher EBJ, Perlow S, Sherman L. Preference reversal and self-control: choice as a function of reward amount and delay. Behav Anal Lett. 1981;1:43–51.
Beeby E, White KG. Preference reversal between impulsive and self-control choice. J Exp Anal Behav. 2013;99:260–76.
Krebs CA, Anderson KG. Preference reversals and effects of d-amphetamine on delay discounting in rats. Behavioural Pharmacol. 2012;23:228–40.
Ito M, Asaki K. Choice behavior of rats in a concurrent-chains schedule: amount and delay of reinforcement. J Exp Anal Behav. 1982;37:383–92.
Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–46.
Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–92.
Kirby KN, Herrnstein RJ. Preference reversals due to myopic discounting of delayed reward. Psychological Sci. 1995;6:83–89.
Sozou PD. On hyperbolic discounting and uncertain hazard rates. Proc R Soc B. 1998;265:2015–20.
Blanchard TC, Pearson JM, Hayden BY. Postreward delays and systematic biases in measures of animal temporal discounting. Proc Natl Acad Sci USA. 2013;110:15491–96.
Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev 2019;99:2115–40.
Pickens R, Meisch RA, Thompson T. Drug self-administration: an analysis of the reinfrocing effects of drugs. In: Iversen LL, Iversen SD, Solomon SH, editor. Handbook of psychopharmacology: drugs of abuse. New York: Plenum Press; 1978. p. 1–37.
Yokel RA. Intravenous self-administration: response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. p. 1–33.
Wise RA. Intravenous drug self-administration: a special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. p. 117–41.
Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacol (Berl). 2005;180:473–90.
Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, Ahmed SH. Choosing under the influence: a drug-specific mechanism by which the setting controls drug choices in rats. Neuropsychopharmacology. 2016;41:646–57.
Freese L, Durand A, Guillem K, Ahmed SH. Pre-trial cocaine biases choice toward cocaine through suppression of the nondrug option. Pharm Biochem Behav. 2018;173:65–73.
Minogianis EA, Shams WM, Mabrouk OS, Wong J-MT, Brake WG, Kennedy RT, et al. Varying the rate of intravenous cocaine infusion influences the temporal dynamics of both drug and dopamine concentrations in the striatum. Eur J Neurosci. 2019;50:2054–64.
Goeders NE, Smith JE. Intracranial self-administration methodologies. Neurosci Biobehav Rev. 1987;11:319–29.
Carlezon WA, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacol (Berl). 1995;122:194–97.
Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science 1983;221:773–75.
Rodd-Henricks ZA, McKenzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered in the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Therapeutics. 2002;303:1216–26.
Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–66.
Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature. 2018;564:366–71.
Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7.
Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62.
Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacol (Berl). 2007;194:127–37.
Lüscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–7.
Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–84.
Guillem K, Ahmed SH. Preference for cocaine is represented in the orbitofrontal cortex by an increased proportion of cocaine use-coding neurons. Cereb Cortex. 2018;28:819–32.
Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–52.
Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–27.
de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31.
Rung JM, Peck S, Hinnenkamp JE, Preston E, Madden GJ. Chaging delay discounting and impulsive choice: implications for addictions, prevention, and human health. Perspect Behav Sci. 2019;42:397.
Ahmed SH. “A walk on the wild side” of addiction: the history and significance of animal models. In: Pickard H, Ahmed SH, editors. The Routledge handbook of philosophy and science of addiction. New York: Routledge; 2019. p. 192–204.
Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3:e2846.
Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77:903–11.
Acknowledgements
We thank Christophe Bernard, Mathieu Louvet and Eric Wattelet for administrative assistance. We also thank Drs. Karine Guillem, Magalie Lenoir, Hanna Pickard and Youna Vandaele for their helpful comments on a previous version of the manuscript. Finally, we would like to thank the reviewers for their constructive criticisms.
Author information
Authors and Affiliations
Contributions
SHA conceived the work. SHA, LC, PG contributed to the design of the experiments. LC and SHA performed the systematic analyses of the literature. LC, PG, AD, CVM carried out the experiments. LC, PG, AD collected the experimental data. SHA, LC analyzed the data. SHA wrote the paper. All authors reviewed content and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Canchy, L., Girardeau, P., Durand, A. et al. Pharmacokinetics trumps pharmacodynamics during cocaine choice: a reconciliation with the dopamine hypothesis of addiction. Neuropsychopharmacol. 46, 288–296 (2021). https://doi.org/10.1038/s41386-020-0786-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0786-9
This article is cited by
-
Assessing behavioral reallocation after acute environmental manipulations using an asymmetric cocaine versus sucrose choice task in male and female rats
Psychopharmacology (2026)
-
A preliminary study of the physiological and perceptual effects of GLP-1 receptor agonists during alcohol consumption in people with obesity
Scientific Reports (2025)
-
Acquisition of cocaine reinforcement using fixed-ratio and concurrent choice schedules in socially housed female and male monkeys
Psychopharmacology (2024)
-
The importance of choice and agency in animal models of addiction
Journal of Neural Transmission (2024)
-
Rats choose alcohol over social reward in an operant choice procedure
Neuropsychopharmacology (2023)